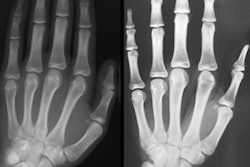
Bone age has typically been determined manually from hand x-rays by radiologists or pediatric endocrinologists, but this method is subjective and prone to reader variability. Danish company Visiana believes its automatic bone age assessment software can do the job better and faster.
Visiana's BoneXpert software provides automatic age analysis from standard hand radiographs in five seconds. And a number of research studies have shown Visiana's BoneXpert software to be more accurate and more precise than manual bone age ratings, according to CEO Hans Henrik Thodberg, PhD.
"There is no need of an operator with experience in bone age rating, and BoneXpert is intended to be used in all clinical situations where bone age is used today," he said.
Formed in 2004, Visiana has focused on developing a method for automated image analysis with the potential for computer-aided detection (CAD), Thodberg said.
"The vision is that combining advanced mathematical modeling, powerful computers, and large databases of images will lead to new CADlike products that will improve patient care and ease the work of radiologists," he said. "It is in particularly the area of quantitative image analysis that Visiana sees the largest advantage, because while the human eye excels in qualitative image interpretation, it is less suited to work as a measuring device."
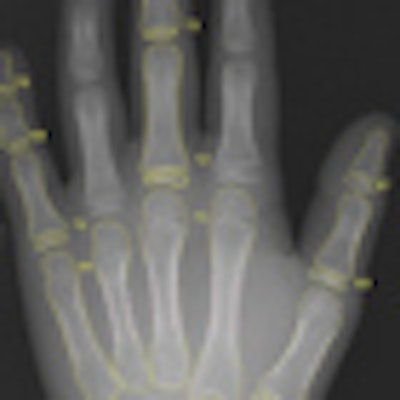
BoneXpert determines the bone age of 13 bones and forms an overall average using the Greulich-Pyle bone age standard. Because that standard is based on pre-World War II Caucasian children, however, BoneXpert also computes ethnicity-dependent bone age standard deviation scores to enable clinicians to assess a child's maturation, Thodberg said.
In addition to bone age measurement, BoneXpert predicts a child's expected adult height based on his or her bone age and current height. The software also generates a bone health index score, which is calculated after performing 100 independent measurements of cortical bone thickness in the metacarpals.
BoneXpert has received the European CE Mark and is used daily at Copenhagen Rigshospitalet, Amsterdam Academic Medical Center, and Leiden University Medical Center; each of these hospitals analyzes 700 to 1,600 images per year with BoneXpert, Thodberg said.
Also, Danish diabetes care company Novo Nordisk has deployed BoneXpert inside its electronic patient registry for growth hormone treatment. Pediatric endocrinologists can upload DICOM images to the registry for analysis by BoneXpert, he said.
At the three hospitals where it's in daily use, BoneXpert is installed on a PC as a standalone Windows program. Images are sent from PACS to the PC via DICOM C-Store protocol. Users select an image from a list of received images, and analysis is completed in five seconds, Thodberg said.
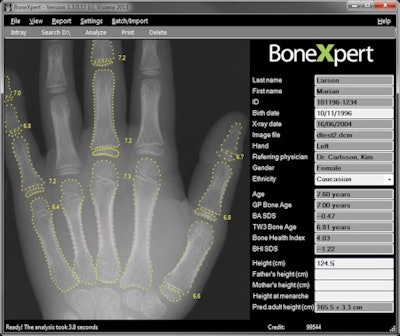 |
| BoneXpert screenshot, courtesy of Hans Henrik Thodberg, PhD. |
Due to some users requesting tighter integration of BoneXpert with PACS, Visiana will introduce a BoneXpert PACS plug-in at the upcoming European Congress of Radiology (ECR) in Vienna in March, Thodberg said. The meeting will also mark Visiana's exhibitor debut at a radiology conference.
Installed on the PACS hardware, the plug-in is activated -- either automatically or by a single operator mouse click -- when a bone age hand x-ray is received. A new image is then created in the same study, Thodberg said.
"There is no [additional] user interface, but simply an additional image with the results embedded as an annotation overlay or a structured report," Thodberg said.
Visiana licenses BoneXpert to users on a pay-per-analysis basis. Each analysis costs 10 euros and includes bone age determination, bone health index calculation, and adult height prediction, he said.
The company has sold BoneXpert directly to hospitals since 2009, but Visiana is also now seeking potential OEM partnerships with PACS vendors and other firms to reach a larger market, Thodberg said.
Visiana also plans to bring BoneXpert to markets other than Europe. In the U.S., the firm has held meetings with the Food and Drug Administration (FDA), Thodberg said.
"To avoid the need of a costly [premarket approval application], the intended use will be less far-reaching than for the CE-Marked European product," he said. "So initially, clearance will be sought for assisting the radiologist in the bone age assessment."
Having recently completed a study involving 6,000 children in five provinces in China, Visiana is also targeting the Chinese market, Thodberg said.