VIENNA - A new digital radiography (DR) system designed for developing markets and a recently acquired extremity MRI scanner are among the highlights in the European Congress of Radiology (ECR) booth of multimodality vendor GE Healthcare of Chalfont St. Giles, U.K.
Brivo DR-F represents the latest introduction in the company's Brivo line of products for price-conscious markets. The unit is best suited for developing countries such as China and those in Eastern Europe where DR currently isn't being used, and it carries a price point about half that of GE's top-of-the-line Discovery DR systems.
Brivo DR-F employs a single DR detector with a tube stand for 180º rotation and a wide, floating table. Approximately 100 systems have been installed since the product began shipping six months ago, with the majority installed in China, according to GE. The product isn't being sold in the U.S.
In the world of MRI, GE is emphasizing the ONI MSK Extreme line of scanners that it acquired with its purchase of ONI Medical Systems in November 2009. The systems are available at 1.5-tesla and 1-tesla field strengths and are optimized for extremity imaging of the knee, foot, ankle, elbow, hand, and wrist.
GE is also touting clinical results from an installation in the Czech Republic that's using one of its 3-tesla scanners for intraoperative scanning. Clinicians at the Central Military Hospital in Prague have sited the magnet next to an operating room, and patients can be shuttled from the OR to the scanning suite on a patient table mounted on rails.
Use of the scanner enables brain surgeons to confirm surgical interventions such as performing biopsies and catheter insertions while keeping patients under anesthesia, and the MRI scanner can be used for general imaging applications when it's not needed for surgical use. More than 300 patients have been treated with MR assistance, and the technique has resulted in the continuance of surgeries once thought complete in 36% of cases.
Other MRI highlights include Brivo MR355, a budget-priced MRI scanner for developing markets that was introduced at the 2010 Arab Health Congress in January and is awaiting CE Mark certification and U.S. 510(k) clearance. Optima MR450w is a wide-bore scanner with 70 cm, while Optima MR360 1.5T is designed for emerging markets.
Finally, GE is touting MR-Touch, the MR elastography application introduced at the 2009 RSNA show, as well as Signa HDxt Optima Edition, an upgrade package that includes new diagnostic tools and a new user interface already in use on the high-end Discovery MR platform.
In women's health, GE is spotlighting the Senographe Essential e full-field digital mammography (FFDM) platform launched at the 2009 RSNA show, as well as a 3-megapixel monitor and a TechInsight application for quality assurance by radiographers/radiologic technologists. Another highlight is an image manipulation tool called Pvi that produces optimal visualization of fatty, dense, and very dense tissue, such as implants, enabling improved clinical confidence and productivity, according to the company.
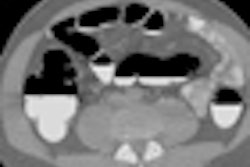
In the realm of CT, a major highlight is GE's low-dose image reconstruction algorithm, ASIR (adaptive statistical iterative reconstruction), as well as SnapShot pulse prospective gating cardiac technique. The company is displaying cardiac images acquired at around 1 mSv of radiation dose, and GE reports that some sites are using ASIR exclusively for all scanning.
GE is also featuring lesion characterization using the Gemstone spectral imaging (GSI) protocol on its high-end Discovery CT750 HD scanner, which can be used to differentiate enhancing versus nonenhancing lesions on contrast exams. The company is also emphasizing a metal artifact reduction (MAR) protocol, which reduces artifacts in CT studies of patients with implanted hardware such as orthopedic devices.
In PET/CT, on tap are dual-detector capabilities and an enhanced application suite for its Discovery PET/CT 600 series scanners, as well as Discovery PET/CT 690 with BrightSpeed, a PET/CT system outfitted with a BrightSpeed Elite CT component. GE also announced the first commercial installation, at Rambam Hospital in Israel, of the Discovery NM/CT 670 SPECT/CT system, which was first introduced in October 2009. The product is awaiting 510(k) clearance in the U.S.
Finally, in ultrasound, GE is touting the Venue 40 compact scanner designed for bedside applications such as needle-guidance procedures and surgical procedures. Other highlights include the Logiq E9 scanner, Breakthrough 2010 upgrade package, and Vscan miniaturized ultrasound unit.
By Brian Casey
AuntMinnie.com staff writer
March 8, 2010
Related Reading
GE introduces updated enterprise archive at HIMSS, March 5, 2010
GE settles with Omniscan critic, February 26, 2010
GE, Intel to study home monitoring, February 24, 2010
GE's Vscan to be used at Olympics, February 19, 2010
GE launches Vscan compact ultrasound, February 16, 2010
Copyright © 2010 AuntMinnie.com