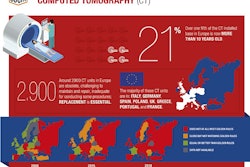
The European Coordination Committee of the Radiological, Electromedical and Healthcare IT Industry (COCIR) has filed a response to a draft publication by the European Commission (EC) that seeks to harmonize medical regulations in Europe.
The EC document on medical device and in vitro diagnostic regulations standardization fails to address key concerns, including a lack of product-specific standards and unnecessarily restrictive deadlines for compliance, according to COCIR.
"The situation around harmonization of standards has deteriorated over the last decade," COCIR wrote in its document. "The medical device sector is only one, though dramatic, example of how the European Commission's legalistic implementation of Regulation 1025/2012 on European standardization could risk the full development of a European single market. This draft standardization request also clearly shows that not all elements essential for the implementation of the Medical Device Regulation will be ready by 26 May 2020."