Radiopharmaceuticals firm Theragnostics is highlighting the results of a phase I clinical trial evaluating the safety of PET/CT using a Ga-68 THP-PSMA tracer in patients with prostate cancer.
The study, published online on 6 October in the Journal of Nuclear Medicine, included 14 patients with biopsy-proven adenocarcinoma of the prostate who were divided into two groups: the first underwent prostatectomy, while the second underwent PET/CT with Ga-68 THP-PSMA as well as an exam with the traditional tracer, Ga-68 HBED-PSMA-11. Researchers then compared the two groups' results.
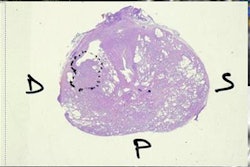
Dr. Michael Hofman of Peter MacCallum Cancer Centre in Melbourne, Australia, and colleagues found that Ga-68 THP-PSMA did not cause any adverse events among patients who had the PET/CT exams, and the tracer's uptake correlated with histopathology results following prostatectomy in the first group.
Ga-68 THP-PSMA can be manufactured quickly using a Ga-68 generator and cold-kit vial, which could allow for rapid clinical adoption and increase the access of PET to prostate cancer patients, Theragnostics said.