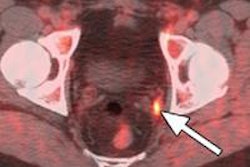
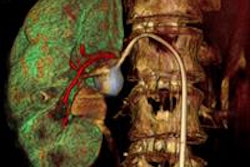
Blue Earth Diagnostics has begun a trial with its PET radiopharmaceutical fluciclovine (F-18) for the management of patients with recurrent prostate cancer.
The trial is being conducted at six facilities: Oxford University Hospitals National Health Service (NHS) Foundation Trust, University College London, Kings College London, the Royal Marsden NHS Foundation Trust, the Leeds Teaching Hospitals NHS Trust, and the Greater Glasgow Health Board.
F-18 is currently under review by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency.