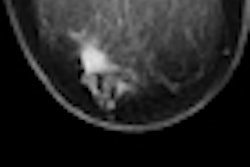
Four year outcomes of early stage breast cancer patients who underwent accelerated partial breast irradiation (APBI) brachytherapy treatment administered with a strut-based device were presented at the World Congress of Brachytherapy, held in Barcelona last week.
Dr. Catheryn Yashar, chief of breast and gynecological radiation services at the Moores Cancer Center of the University of California San Diego in La Jolla, presented data about 50 women who have been followed for at least four years. Only one patient had a recurrence, which is comparable to outcomes for patients who had whole breast irradiation.
The study pertains specifically to patients who received their breast brachytherapy treatment with the use of a strut-adjusted volume implant (SAVI) multicatheter single entry device (Cianna Medical) at one of three cancer treatment centers. In addition to the Moores Cancer Center, these included the Arizona Breast Cancer Specialists in Phoenix, and 21st Century Oncology in Fort Myers, Florida.
The patients received a dose of 340 cGy administered in 10 fractions over five days. Patients were graded on disease status, cosmesis, hyperpigmentation, seroma, induration, erythema, fibrosis, telangiectasias, fat necrosis, and breast symmetry.
None of the patients experienced any grade 3 late toxicities. The most common grade 1 and grade 2 toxicity was fibrosis, which 18% and 10% of the patients experienced. One patient each experienced grade 2 erythema, telangiectasias, and breast pain. After fibrosis, the next three most frequently reported grade 1 toxicities were induration (12%), breast asymmetry (10%), and telangiectasias (10%).