New software will soon provide automatic tumor measurements for gold-standard therapy planning at one of Spain's top hospitals. In the longer term, instant evaluation of tumor necrosis in follow-up should be feasible, for timely treatment tailoring once therapy is under way.
Three months postinstallation, Median Technologies' Lesion Management Solutions (LMS) software has already vastly improved workflow in a medical center that boasts six CT scanners, five MRI units, one PET system, and 23 ultrasound machines, according to radiologists at Hospital Universitari i Politècnic La Fe in Valencia.
As part of the country's largest ever PACS tender, managers at La Fe selected Agfa Healthcare Spain as the PACS provider and chose to integrate the LMS software into the system, which is accessible from any PACS workstation at the hospital. Dedicated to oncology and pulmonology, the software is currently being used in clinical routine in chest and abdominal radiology and oncologic imaging.
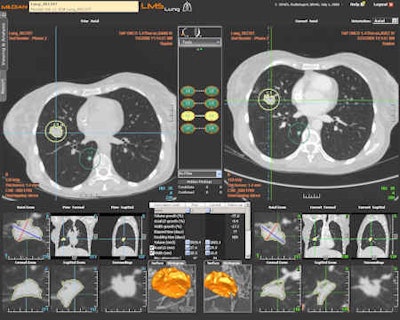 Follow-up of a pulmonary mass with the LMS platform (oncology imaging). Besides oncology imaging specialists, chest and abdominal radiologists will in the future use the software for pretreatment measurements after first detection of tumors. All images courtesy of Median Technologies.
Follow-up of a pulmonary mass with the LMS platform (oncology imaging). Besides oncology imaging specialists, chest and abdominal radiologists will in the future use the software for pretreatment measurements after first detection of tumors. All images courtesy of Median Technologies."LMS runs within PACS to find the same lesions at second examination follow-up, and measure and chart any changes. This saves the radiologist time; each follow-up CT evaluation process is 10 to 15 minutes faster than without using LMS," said Dr. Luis Martí-Bonmatí, La Fe's director of clinical imaging and president elect of the European Society of Gastrointestinal and Abdominal Radiology. "My feeling is that this automatic evaluation is more accurate and reliable as it doesn't suffer from interobserver variation."
Although LMS to date has been primarily used in oncology follow-up, the hospital management is considering employing it in pretreatment evaluation of lesions, which will involve using the size and volume of lesions to determine pretreatment baseline measurements and aid in treatment planning. At the moment these measurements are determined using relatively simple techniques such as gauging the diameter and distance using manual calipers, but this move toward automatic evaluation with LMS is set to take place in the next three to four months.
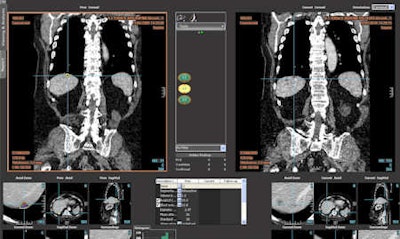 Follow-up of a hepatic lesion with the LMS platform (abdominal radiology) now in routine practice at La Fe Hospital.
Follow-up of a hepatic lesion with the LMS platform (abdominal radiology) now in routine practice at La Fe Hospital.In a further step, the hospital aims to use the software in the evaluation of tumor necrosis to follow tumor response to therapy. To do this, methods to properly measure enhancement, thus automatically measure viable tumor, will need to be explored and established.
"It is important not to change treatment purely due to volume measurements. A tumor might have less volume but huge vascularity, or it might shrink but the vascularity remains, necessitating a change or continuation of treatment. Alternatively it may not shrink but still die," Martí-Bonmatí said.
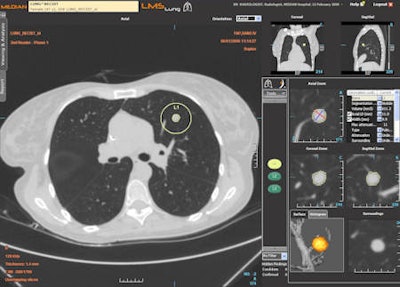 CAD of pulmonary nodules with the LMS platform (pulmonology).
CAD of pulmonary nodules with the LMS platform (pulmonology).At present, vascularity is evaluated visually or by measuring the change in Hounsfield units in follow-up. Automatic evaluation will require the development of new software based on physiologic computing models of vascularization. Trials in evaluation of post-treatment vascularity could start as early as next year.
"Median is thrilled to work with such a reputed institution. After such successful LMS deployment, Median already considers La Fe as a reference site," said Gérard Milhiet, executive vice president and co-founder of Median Technologies. "In the near future, La Fe is also likely to showcase to pharma groups the benefits of using LMS for clinical trials to study the efficacy of new oncology drugs or new therapeutic strategies."