Imaging is central to the unprecedented wave of medical specialties converging on cancer care. But it will take more than what today's radiologists are trained for to better understand cancer's mysteries, according to global cancer imaging pioneer Dr. Hedvig Hricak, PhD.
Radiologists, geneticists, medical physicists, and radiation oncologists -- hybrid multidisciplinary teams will be needed for the challenge of cancer assessment and treatment in a world in which every cancer is different. Unfortunately, no one is training the doctors of today, let alone tomorrow, about the technologies they will need to master, said Croatian-born Hricak in a keynote address at this week's American Society for Radiation Oncology (ASTRO) meeting.
"Our problem today is not the amount of smart probes or radiotracers," she said. "Our biggest challenge is that we really do not have a sufficient number of oncology imaging people who truly understand what radiation therapy is and how cancer should be imaged."
Hricak chairs the radiology department at Memorial Sloan Kettering Cancer Center and served as president of RSNA 2009. She received the European Society of Radiology (ESR) Gold Medal at ECR 2012.
An unprecedented convergence
Today's medical practitioners are witnessing an unprecedented convergence of the life sciences, physical sciences, and engineering, and much of this has happened because of advances in imaging, she said. Since the mid-1980s, the "alphabet soup" of imaging modalities -- from CT to MRI to PET -- has become a collection of new hybrid modalities such as PET/MRI.
 Dr. Hedvig Hricak, PhD, from Memorial Sloan Kettering Cancer Center.
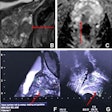
Dr. Hedvig Hricak, PhD, from Memorial Sloan Kettering Cancer Center.Imaging's advances continue in oncologic imaging, especially in MRI, Hricak said. MRI of prostate cancer allows exquisite definition of soft-tissue morphology. And because anatomy is so well-defined, radiologists can be quite precise about tumor localization, such as tumor in the peripheral zone, anteriorly located tumor in the transition zone, or tumor in the central zone or at the base that has different biological properties than at other locations.
"You should think about how to treat tumor differently in those locations," she said.
What does it mean when a prostate cancer tumor recurs at the original site? Is it radiosensitive? What's the best approach for targeting it aggressively? New technologies give us many answers, but decoding the best options takes increasingly advanced skills.
"The higher the technology, the greater complexity and greater expertise is required to get the best out of a technology," Hricak said.
In radiation oncology and radiology alike, the best technique for the job depends on the organ and the cancer. In MRI alone, for example, prostate cancer requires diffusion-weighted MRI, while hepatocellular carcinoma requires portal-venous-phase contrast-enhanced T1-weighted MRI, and breast cancer takes delayed postcontrast T1-weighted MRI. And that's just the beginning.
MRI is superb for detecting prostate cancer, but detection is dependent on both volume and Gleason score, she said. On T2-weighted MRI, there is a significant difference in tumor volumes smaller or larger than 1 cc, and there are significant differences in the detection of low- and high-grade tumors.
Diffusion-weighted imaging (DWI) is better than T2-weighted MRI, and tumor volume assessments are also more accurate and offer quantitative information that correlates with prostate cancer aggressiveness as measured in Gleason score. But about half of small tumors can't be seen on DWI, and even lower-grade tumors may lead to metastasis, Hricak said.
Fortunately, another MRI measure, the mean DWI apparent diffusion coefficient (ADC) value, is a strong predictor of tumor aggressiveness. But that's yesterday's news, according to Hricak. ADC histogram analysis has been found to correlate significantly better with Gleason score -- much better than mean ADC.
And lately, texture analysis of DWI or T2 images in postprocessing has been shown to be another way of assessing tumor aggressiveness by assessing tissue heterogeneity, and it doesn't matter if the tumor is large or small.
"Because of this complexity, to get the best out of technology we need to work together, and 'we' means radiation oncology, medical physics, and diagnostic radiology," she said. "Only then are we going to really understand the advantages and the limitations."
Genome's game changer
In molecular imaging, the sequencing of the human genome in 2003 opened new doors to disease assessment. For oncology detection and treatment, the understanding of tumor heterogeneity and its vast implications has been key knowledge gleaned from this work, Hricak said. In 2013, Vogelstein et al showed that genetic heterogeneity can occur within a single primary tumor, between two metastases, within two metastatic lesions, and, of course, between patients.
In this environment, biologic information is essential for understanding treatment options, she said. Two tumors representing a single cancer phenotype may look and behave very differently when seen through a genetic lens, and they require different treatment and assessment strategies. Thus, the traditional anatomy lessons on which our specialties are based are changing radically. What does this knowledge mean in practice?
"We need to develop new biosignature tools to visualize tumor heterogeneity and identify key drivers of tumor response," Hricak said. "And it's about time we really meet together to have a paradigm shift from one size fits all to radiation treatment that's predictive, prognostic, and personalized -- the precision legacy that brings tumor morphology and biology and biomarker-driven, evidence-based radiation therapy."
The case of metastatic breast lesions highlights how different modalities see the same thing differently, Hricak said. In one patient, FDG-PET shows homogeneous high uptake as measured by standardized uptake values. In the same patient, copper-64 trastuzumab receptor imaging reveals significant intratumoral heterogeneity between two lesions. Precision biopsy shows HER2 gene copies in the area of high receptor uptake. It adds up to mixed tumor response in the setting of intermetastatic heterogeneity, and an important lesson for treatment, she said.
"We need to align our smart probes with the questions we ask," she said. One question to address might be which lesion to biopsy in a patient with many lesions revealed by different radiotracers. What is the total tumor burden, and which are more aggressive? In the setting of continuous tumor differentiation, when and how many tumors do you rebiopsy? How and when do you modify treatment, and when do you escalate or reduce the dose?
In prostate cancer, the FDG-PET image of glycolosis can look very different from results of an androgen receptor probe on FDG-PET/CT. Another smart probe approach is C-labeled substrates and their metabolic products, which allow for assessing tumor aggressiveness and response. One study used C-labeled pyruvate to show lactate conversion in lymphoma patients treated 20 hours earlier with the chemotherapy agent etoposide, handily separating responders from nonresponders. Today's experimental technologies need to be incorporated into tomorrow's practices, Hricak said.
The answer to metastatic disease questions is imaging, and that imaging has to include phenotype to interrogate the molecular pathogenesis of cancer, as well as looking at different types of smart probes, she said. There is much more to be learned about the biology of metastatic disease, and there are already a wealth of imaging tools, though few have been approved by the U.S. Food and Drug Administration (FDA), to help complete the task. C-choline PET/CT can be used to differentiate lymph node metastases. Gallium-68 prostate-specific membrane antigen (PSMA) PET/CT can be used to detect lymph node metastases. And even without contrast or advanced radiotracers, diffusion-weighted MRI with multiple B values can highlight biologic heterogeneity.
From lymphoma to prostate, head and neck, and colon cancer, researchers are eagerly awaiting the results of many trials to evaluate the new ways of assessing tumor treatment response, she said. Hundreds of smart probes are being investigated.
"Knowing the extent of disease is the essence of our future," she said.
Today's investigators don't lack smart probes or radiotracers in development. Memorial Sloan Kettering alone has 32 radiotracers under investigation (none FDA-approved), Hricak said. But while the technology is here, there are big challenges -- in particular, the lack of people who know how to use it. There simply aren't enough experts in oncologic imaging who can put it all together, and there is little in the way of training prospects that would change the situation in the near future.
"It is not a part of our resident programs; it is not examined," she said. "We just don't have enough experts so we can all truly work together. Multidisciplinary teams and treatment planning are luxuries that very few places have," and radiologic and oncologic imaging are not recognized as having value.
Advances in oncologic imaging and therapy have shown that every cancer is different, and each requires a different approach, Hricak said. How will treatment planning and pretreatment diagnosis be conducted, and how will response be measured? The questions are complex and require all disciplines to work together. They will also require the FDA to approve the new probes that researchers have created.