Swedish nuclear medicine software developer Hermes Medical Solutions has received U.S. Food and Drug Administration (FDA) clearance for new software for reconstructing and processing hybrid nuclear medicine (NM) studies.
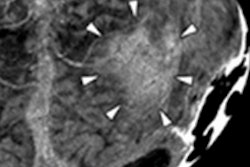
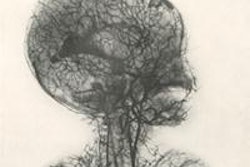
The company's Hybrid Viewer display software can be used to reconstruct and process SPECT and SPECT/CT exams, regardless of gamma camera manufacturer. The application uses a reconstruction algorithm that corrects for photon attenuation using either a CT or radionuclide scan-based attenuation map.
It also features correction for depth-dependent collimator response using calculated collimator point-spread functions, as well as scatter correction using the Hermes Monte Carlo simulator. Nuclear medicine processing protocols range from renogram analysis to planar gated cardiac analysis, the company said.
In addition, the advanced processing that was previously available in the Hermes "classic" environment is now updated and integrated into Hybrid Viewer, and new features such as planar and dynamic image registration and customized workflow templates are now built into the user interface.