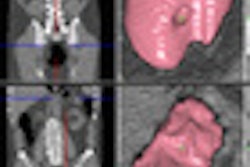
Researchers at University College London and software developer Medicsight in the U.K. have validated and improved a technique that automatically registers prone and supine images on CT colonography (CTC). They believe the algorithm, developed by Medicsight, is robust enough for daily clinical use.
Use of the algorithm, which is investigational and not commercially available, could also save reading time and potentially reduce reader errors, the group found. In data from 49 patients with 66 polyps, prone and supine CTC images were matched with registration errors of less than 20 mm (mean) or 12 mm (median), providing a direct match to polyp location in more than half of the CTC cases registered.
Moreover, neither excess fluid nor the presence of collapsed colonic segments reduced accuracy significantly, making the algorithm practical for daily clinical use, the investigators said in a presentation at the 2011 RSNA meeting in Chicago.
 |
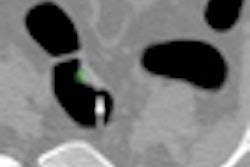
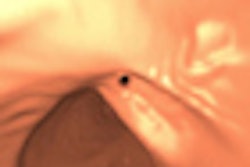
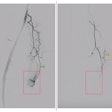
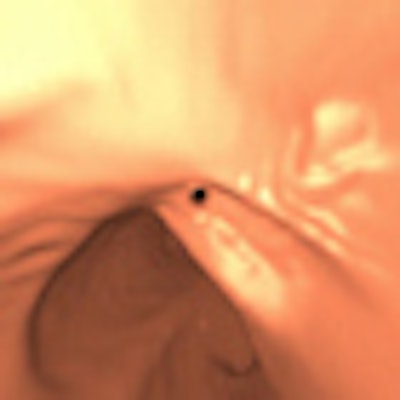
| In the prone-supine registration example above, which received a score of 4 for "near miss," the endoluminal field-of-view is centered on the registration prompt (black spot), and the polyp is evident but not directly marked (white circle). All images courtesy of Greg Slabaugh, PhD. |
CTC is normally acquired in two positions, which aids in distention of the colon and helps distinguish mobile colonic residue from colonic pathology, said Greg Slabaugh, PhD, from Medicsight, in his talk. Unfortunately, the colorectum undergoes considerable deformation during patient positioning, and it's hard to match things up again.
"Manually establishing the corresponding locations in opposing datasets can be challenging, potentially prolonging interpretation time and possibly engendering reader error," Slabaugh said. The researchers addressed this problem with a novel prone-supine registration algorithm to facilitate interpretation. Automating the task of matching prone and supine virtual colonoscopy datasets will aid polyp characterization and facilitate interpretation, he said.
Slabaugh, along with Darren Boone and colleagues, aimed to validate the registration algorithm in terms of its clinical usefulness, Slabaugh said, noting that proper validation requires the use of CTC cases that are generalizable to daily practice, and data that have not been used for developing the software.
Several other prone-supine registration schemes have been developed, he said. However, because they are generally based on centerline matching, they are less robust and typically must be validated on perfectly prepared CTC cases or tested on data for which the algorithm was developed. This means that their accuracy is difficult to extrapolate to clinical practice. The algorithm developed for the study is different in that it's designed for use in real-world CTC practice, he said.
Slabaugh listed three main components of the algorithm:
- Initialization: Fold similarities detected using a Markov random field model, as described in Hampshire et al
- One-to-one conformal mapping: Cylindrical registration based on surface curvature, as described in Roth et al
- Algorithm trained on in-house CTC data: No further development from test data after initiation of validation study
A graph-cut method was used to detect haustral folds and create endoluminal image pairs from prone and supine CTC datasets. The Markov random field model provided haustral fold alignment, using image intensity and neighborhood differences. This process automatically initializes a surface-based registration algorithm that uses nonrigid deformation of cylindrical parameterizations to achieve one-to-one spatial correspondence, Slabaugh explained.
Test data were gleaned from publicly available National CT Colonography Trial (ACRIN 6664) results to validate the data. After excluding data where polyps were not visible in both datasets and images were corrupted, the algorithm was tested using virtual colonoscopy data from 49 patients comprising 66 polyps (38 were 10-13 mm in size; 28 were 6-9 mm). Polyps larger than 30 mm were excluded from analysis.
Eight of the cases were fully distended, while five had multiple colonic collapses. None had been used for algorithm development. A radiologist with experience in more than 500 validated datasets provided polyp coordinates and a further 1,175 reference points distributed over the endoluminal colonic surfaces, Slabaugh said.
Automated registration places a prompt in the opposing dataset and centers both 2D and 3D views on this prompt, he said. To test the method polyp by polyp, the researchers selected a prone or supine polyp, and using the algorithm placed a prompt in the opposing dataset. First they computed the 3D error between the prompt and the true polyp, and defined a 3D conspicuity score (scale of 1 to 5, with 5 being best) and a 2D conspicuity score as well. Then they calculated the gross error between "anticipated" and "real" polyp epicenters from 3D volumes, Slabaugh said.
For the 3D registration, the mean error was 19.9 mm and the median error was 11.9 mm, Slabaugh reported. There was no significant difference in median registration error when comparing the segmental location in cases with at least one luminal collapse or in cases with excess residual fluid, he said.
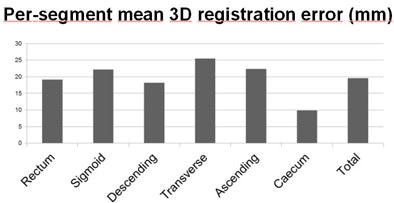 |
| The mean registration error of 19.9 mm varied only moderately between colonic segments. |
Per-segment polyp distribution showed no significant difference compared to the overall ACRIN study (p = 0.81) in terms of percentage of polyps in the luminal view. In all, 82.7% of polyps were either marked or immediately visible in the endoluminal view.
In cases with inadequate bowel cleansing:
- 55% (n = 26) were correctly marked in the validation sample
- 52% (n = 1,313) were correctly marked in the full ACRIN dataset
In cases with at least one region of complete luminal collapse:
- 47% (n = 31) were correctly marked in the validation sample
- 49% (n = 51) were correctly marked in the total publicly available ACRIN archive
The researchers found similar results for the 2D polyp score, with 77.3% of polyps either marked directly or within 15 mm on the 2D axial view.
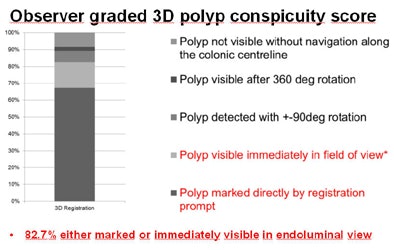 |
| The mean conspicuity scores used to grade registration accuracy showed that in both 3D (above) and 2D (below) results, the algorithm marked most polyps directly on the opposing dataset, with very few registration failures even when datasets contained collapsed segments or excess colonic residues. |
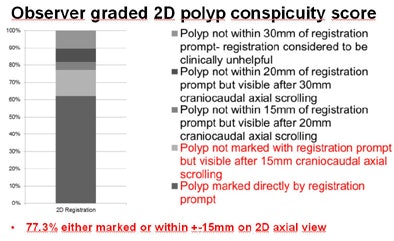 |
"The validation sample that we used reflects CTC in daily practice in terms of polyp distribution, cleansing, and distention," he said. "For over 75%, the prone-supine registration brings the observer to a location where the polyp is either within the immediate view in 3D or within 15 mm in 2D. In over 50% of the cases, the polyp was directly marked by the algorithm."
Future work will focus on continued refinement and speeding up of the processing, Slabaugh said in response to a question from the moderator.
| Lead author Slabaugh is an employee of Medicsight. |