Contrast-enhanced mammography (CEM) should be considered a second-line tool in the diagnostic and therapeutic process, and when there is limited access to MRI or patients with contraindications to MRI, CEM can serve as a dependable alternative for preoperative breast cancer evaluation, according to new research.
“CEM may serve as a valuable diagnostic tool for patients with pronounced background parenchymal enhancement, likely due to high-density breasts,” noted Dr. Manuel R López de la Torre Carretero and colleagues from the department of radiology at Clínica Universidad de Navarra in Pamplona, adding that MRI is more susceptible to background parenchymal enhancement.
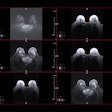
 Case of a multicentric Luminal A invasive ductal carcinoma. While conventional mammography would have detected one lesion, contrast-enhanced mammography detected multicentricity, and this was confirmed by MRI. All figures courtesy of Drs. Manuel R López de la Torre Carretero, Miguel Barrio Piqueras, and Luis Javier Pina Insausti and presented at ECR 2025.
Case of a multicentric Luminal A invasive ductal carcinoma. While conventional mammography would have detected one lesion, contrast-enhanced mammography detected multicentricity, and this was confirmed by MRI. All figures courtesy of Drs. Manuel R López de la Torre Carretero, Miguel Barrio Piqueras, and Luis Javier Pina Insausti and presented at ECR 2025.
In their recent study, breast MRI yielded significantly higher sensitivity, and the area under a curve of CEM exceeded that of MRI due to the higher rate of false positives obtained in MRI exams.
“In the preoperative assessment of malignant breast lesions, a higher rate of false positives (as seen in MRI) is more acceptable than a high rate of false negatives (as seen in CEM), considering the risk of failing to diagnose intermediate-risk tumors with this technique,” they explained in a poster presentation at ECR 2025. “Regarding tumor size measurements, both CEM and MRI demonstrated comparable performance, with a tendency to overestimate lesion size.”
CEM can address the challenges associated with breast MRI, including high false positive rates, long acquisition times, high cost, claustrophobia, and accessibility, the authors pointed out. It uses a dual-energy approach and iodinated contrast material for breast neoangiogenesis assessment, and recombined images showing contrast material uptake can be considered an analogue of MRI subtraction images.
“CEM is considered a faster, cheaper, and more accessible technique to implement as a problem-solving in daily screening, with suggested increased sensitivity for breast cancer detection relative to conventional digital imaging, and in some cases equal sensitivity with improved specificity relative to breast MRI in the diagnostic setting,” they stated.
Study logistics
The Pamplona team’s study aimed to retrospectively evaluate the accuracy of CEM and MRI in preoperative staging of breast cancer. A secondary objective was to determine the accuracy of lesion size measurement with both techniques.
The group focused on confirmed breast cancer cases in patients who underwent both MRI and CEM between September 2017 and November 2023. Two radiologists retrospectively reevaluated all images in both techniques.
CEM was used as a problem-solving technique, complementing digital mammography, digital breast tomosynthesis (DBT), and ultrasound. Breast MRI was performed after histological malignancy confirmation. In cases of neoadjuvant chemotherapy, another preoperative MRI was performed to evaluate the tumor response.
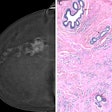
 Case of a unifocal Luminal B invasive ductal carcinoma. Contrast-enhanced mammography shows better accuracy than conventional techniques in detecting these lesions, and this was confirmed by MRI study.
Case of a unifocal Luminal B invasive ductal carcinoma. Contrast-enhanced mammography shows better accuracy than conventional techniques in detecting these lesions, and this was confirmed by MRI study.
MRI studies were performed on a 1.5-tesla scanner in the prone position, with no breast compression, and using a dedicated four-channel breast coil. The MRI protocol included an axial STIR-T2 weighted sequence, an axial diffusion-weighted imaging (DWI) sequence and a dynamic acquisition 3D axial sequence after the administration of 0.1 mmol/kg of gadolinium.
All CEM exams started with an intravenous injection of 1.5 mL/kg of iodinated contrast agent, using an automated rate of 3mL/s followed by a saline flush of 25 mL. At the time of injection, the breast was not compressed to allow for normal tissue perfusion. After a waiting time of 2 min, the breast was compressed, and low (28 and 32 kV) and high energy (49 kV) images were acquired. Recombined subtraction images were formed, highlighting areas of iodine uptake and cancelling signal from normal background parenchymal enhancement.
Summary of results
A total of 139 histopathologically confirmed lesions from 94 women were included in the study. The mean age was 58, with 34 women in the younger subgroup (under 50) and 61 in the older subgroup (over 50).
Histologically, 23 benign and 116 malignant lesions were identified. Among the malignant lesions, 49 Luminal A-invasive ductal carcinoma (A-IDC), 31 Luminal B-invasive ductal carcinomas (B-IDC), and 9 triple negative invasive ductal carcinomas (TN-IDC) were described. Of the malignant lesions, 65 were classified as unifocal, 25 as multicentric, 24 as multifocal, and 2 as bilateral.
Number of malignant lesions (%) | Histological subtype |
49 (41.2%) | Luminal A-invasive ductal carcinoma (A-IDC) |
5 (4.2%) | Luminal A-invasive lobular carcinoma (A-ILC) |
2 (1.7%) | Luminal A-tubular carcinoma |
1 (0.8%) | Luminal A-papillary carcinoma |
31 (26.1%) | Luminal B-invasive ductal carcinoma (B-IDC) |
7 (5.9%) | Luminal B-invasive lobular carcinoma (B-ILC) |
8 (6.7%) | Invasive ductal carcinoma-HER2 (IDC-HER2) |
9 (7.6%) | Triple-negative invasive ductal carcinoma (TN-IDC) |
7 (5.9%) | Ductal carcinoma in situ (DCIS) |
Table shows all histological subgroups of malignant lesions. | |
MRI showed 18 false positives and 4 false negatives, while CEM showed 1 false positive and 22 false negatives. The global sensitivity to detect breast cancer was 96.6% for breast MRI vs 81% for CEM (p = 0.001).
The researchers found a sensitivity of 97.6% for MRI versus 85.4% for CEM (p = 0.06) in the younger women, and a sensitivity of 96.1% for MRI versus 82.9% for CEM (p = 0.075) in the older women. In terms of breast density, MRI had a sensitivity of 96.9% for MRI versus 81.6% for CEM in the high-density subgroup (p = 0.006), showing a higher sensitivity of MRI versus CEM with a general tendency to statistical significance.
Among the histology of the false negatives in each technique, MRI failed to detect 4 lesions (1 ductal carcinoma in situ [DCIS] and 3 Luminal-A). On the other hand, CEM failed to detect a total of 22 tumors, including 5 DCIS (one of them measuring 45 mm), 8 Luminal A, and 9 Luminal B. Both techniques detected all HER2-positive and TN carcinomas.
The authors conceded that this is a retrospective study, and five out of seven cases of DCIS included in the malignant lesions burden showed no microcalcifications, which may introduce selection bias.
“We continue to expand our database and are seeing promising developments in the field, although we are not conducting new research specifically on this topic at the moment,” López de la Torre Carretero, a radiology resident, told AuntMinnieEurope.com on 2 May. “Right now, our next challenge is lesion detection using AI, but we are also exploring related areas that may build upon the findings presented in the ECR poster.”
You can read the full ECR poster here. The co-authors were Miguel Barrio Piqueras, Carlos Delgado Solano, Daniel Alfonso Zambrano, Adolfo Manuel Delgado Brito, José Marlon Rodríguez Ortega, Carmen Mbongo, and Arlette Elizalde, Luis Javier Pina Insausti (principal investigator).