The combination of mammography and supplemental ultrasound delivers better results than relying only on AI-enhanced mammography assessment in screening women with dense breasts, according to research published on 23 July in Radiology.
A team led by Su Min Ha, MD, PhD, from Seoul National University Hospital in South Korea found that while AI improved the specificity of mammography interpretation, mammography plus supplemental ultrasound detected more node-negative early breast cancers that AI-enhanced mammography could not detect.
“AI may help radiologists interpret screening mammography, but it cannot fully compensate for its low sensitivity in women with dense breasts,” Ha and colleagues wrote.
It’s not clear whether AI can best supplemental ultrasound for screening women with dense breasts. Previous AI studies suggest that AI systems can assist radiologists with image interpretation. But others have found that AI may struggle with detecting malignant lesions in dense breasts like human readers do with mammography interpretation.
Ha and colleagues in their retrospective study compared the respective performances of mammography alone, mammography with AI (Lunit Insight MMG version 1.1.7.1; Lunit), and mammography plus supplemental ultrasound for screening women with dense breasts. They also studied the characteristics of detected breast cancers.
The study included data from 5,707 asymptomatic women with an average age of 52.4 years. All women included in the study were over the age of 40. The women underwent mammography plus supplemental whole-breast handheld ultrasound from January 2017 to December 2018 at a primary health care center.
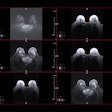
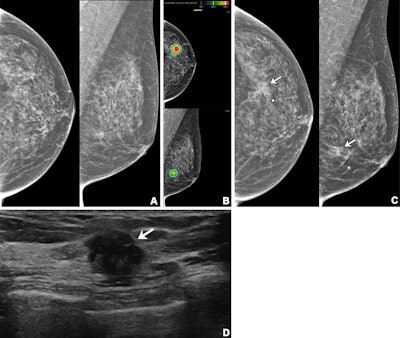 Imaging in a 56-year-old woman with heterogeneously dense breasts (BIRADS C). (A) Left craniocaudal (left) and mediolateral oblique (right) digital mammograms show no abnormality. (B) Left craniocaudal (LCC, top) and left mediolateral oblique (LMLO, bottom) mammograms with retrospectively applied AI (Lunit Insight MMG, version 1.1.7.1; Lunit) show the lesion was given abnormality scores of 97% and 71%, respectively. The color bar indicates pixel-level abnormality scores corresponding to heatmap contour lines. Breast ultrasound performed the same day was considered negative (not shown). (C) Left craniocaudal (left) and mediolateral oblique (right) digital mammograms acquired nine months later show an irregular mass (arrows) in the left breast, which correlates with the heatmap areas scored as 97% and 71% by AI in the earlier screening mammogram (B). The radiopaque round marker denotes the site of the palpable abnormality indicated by the patient. (D) Transverse ultrasound imaging shows an irregular mass (arrow) in the left breast. The patient was treated with breast-conserving surgery, and the mass was proven to be invasive lobular carcinoma. Image courtesy of the RSNA.
Imaging in a 56-year-old woman with heterogeneously dense breasts (BIRADS C). (A) Left craniocaudal (left) and mediolateral oblique (right) digital mammograms show no abnormality. (B) Left craniocaudal (LCC, top) and left mediolateral oblique (LMLO, bottom) mammograms with retrospectively applied AI (Lunit Insight MMG, version 1.1.7.1; Lunit) show the lesion was given abnormality scores of 97% and 71%, respectively. The color bar indicates pixel-level abnormality scores corresponding to heatmap contour lines. Breast ultrasound performed the same day was considered negative (not shown). (C) Left craniocaudal (left) and mediolateral oblique (right) digital mammograms acquired nine months later show an irregular mass (arrows) in the left breast, which correlates with the heatmap areas scored as 97% and 71% by AI in the earlier screening mammogram (B). The radiopaque round marker denotes the site of the palpable abnormality indicated by the patient. (D) Transverse ultrasound imaging shows an irregular mass (arrow) in the left breast. The patient was treated with breast-conserving surgery, and the mass was proven to be invasive lobular carcinoma. Image courtesy of the RSNA.
Five breast radiologists meanwhile conducted sequential reading for mammography alone and mammography with the aid of an AI system. A dedicated breast radiologist also reviewed marks for mammography alone or with AI to confirm lesion identification.
Of the total women, 33 (0.6%) had cancer, which included a median lesion size of 0.7 cm. Of the cancers, 31 were without lymph node metastasis.
The researchers reported that mammography with AI had a higher specificity and lower abnormal interpretation rate compared with mammography alone. However, mammography plus ultrasound outperformed mammography with AI in terms of cancer detection rate and sensitivity.
| Performances of mammography, mammography plus AI, mammography plus ultrasound for screening dense breasts | |||
|---|---|---|---|
| Variable | Mammography alone | Mammography plus AI | Mammography plus ultrasound |
| Cancer detection rate (per 1,000 exams) | 3.3 | 3.5 | 5.6 |
| Sensitivity | 57.6% | 60.6% | 97% |
| Specificity | 94.3% | 95.3% | 77.6% |
| Abnormal interpretation rate | 6% | 5% | 22.9% |
| Positive predictive value (PPV) | 5.5% | 7% | 2.5% |
| Negative predictive value (NPV) | 99.7% | 99.8% | 99.9% |
The team also found that supplemental ultrasound alone helped detect 12 cancers, mostly stage 0 and I (92%). Also, ultrasound had an incremental cancer detection rate of 2.2 per 1,000 exams after negative results, according to mammography with AI.
The study authors called for future studies to focus on the clinical application of AI for women undergoing mammography and supplemental ultrasound, incorporating more clinical and temporal data.
As AI systems become more advanced, verifying improved performance in screening mammography applications will be important, according to an accompanying editorial by Gary Whitman, MD, from the University of Texas MD Anderson Cancer Center, and Stamatia Destounis, MD, from Elizabeth Wende Breast Care in Rochester, NY.
The two added that future studies should emphasize reducing false-negative studies rather than reducing false-positive cases.
“It would be beneficial to evaluate AI performance on a broader scale with studies with more patients, more AI systems, and a longer follow-up period,” Whitman and Destounis wrote. “In addition, in the future, it is likely that breast cancer screening strategies will be tailored for each woman’s risk of developing breast cancer.”
The full study can be found here.