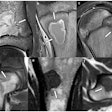
GE HealthCare (GEHC) is highlighting results from a phase I clinical trial for a macrocyclic manganese-based MRI contrast agent.
The trial was conducted at Oslo University Hospital in Rikshospitalet, Norway, and its results were presented at the Contrast Media Research symposium in Oslo. It found that the agent was well tolerated by patients and produced no serious adverse events or dose-limiting toxicities, the company said.
If the agent is cleared by the U.S. Food and Drug Administration (FDA) it would offer an alternative to gadolinium-based contrast agents, according to the firm.
In other GEHC news, the company has joined Thera4Care, a $27.7 million initiative to boost patient access to theranostics that includes 29 partners from European academic and clinical sites, small and medium-sized companies, and patient advocacy groups. The project will increase the use and adoption of radiology-based diagnostics and therapies for a variety of cancers, GEHC said, in the following ways:
- Expanding the European network of copper producing isotope good manufacturing practices (GMP) sites.
- Developing new SPECT-CT imaging scanners.
- Building AI-enabled imaging and multimodal theranostic clinical decision support.
- Enhancing AI-based tumor quantitation by developing a methodology for personalized dosimetry.
Thera4Care is funded by the Horizon Europe framework and is part of the Innovative Health Initiative (IHI), a public-private partnership between the European Union and the European life science industries.