A new gallium-68 PET radiotracer appears effective for predicting higher risk of disease progression and mortality in patients with neuroendocrine tumors, according to a study published on 18 August in the Journal of Nuclear Medicine.
Danish researchers at the University of Copenhagen tested the use of a gallium-68 (Ga-68) NODAGA-E[c(RGDyK)]2 tracer designed to image certain proteins involved in the growth of neuroendocrine tumors (NETs). In results of a phase II trial, they found that high signals of the tracer on PET scans predicted poorer outcomes for patients.
"High tumor uptake of Ga-68 NODAGA-E[c(RGDyK)]2 was associated with a poorer prognosis in patients with [NETs] with a twofold higher risk of progression and sevenfold higher risk of death," wrote first author Dr. Esben Carlsen, a clinical physiology and nuclear medicine fellow, and colleagues.
NETs occur almost everywhere in the body, but they are most common in the gastrointestinal tract, the pancreas, and the lungs. Survival rates for patients depend on how aggressive the tumors (grades 1-3) are and the stage at which they are diagnosed.
Research has shown that the activity of integrin alpha-v beta-3 (avb3) receptors on NET cancer cells help stimulate growth of the tumors, and these receptors have become promising targets for new therapies to prevent the growth of cancer. Imaging the effects of these new therapies will be key moving forward, the authors wrote.
The group first synthesized the radiotracer in 2014 and subsequently established in animal studies and first-in-human trials that Ga-68 NODAGA-E[c(RGDyK)]2 reveals avb3 activity in NET tumors on PET imaging. In this study, they report results from research of the radiotracer in a group of patients with NETs of all grades, with the aim of assessing its prognostic value.
Between December 2017 and November 2020, the researchers recruited 99 patients with NETs for PET imaging at Copenhagen University Hospital using Ga-68 NODAGA-E[c(RGDyK)]2. Whole-body PET scans (Biograph mCT, Siemens Healthineers) were acquired 45 minutes after intravenous administration of the radiotracer.
Patients predominantly had low-grade small intestinal or pancreatic tumors and metastatic disease (93%), and most were being treated with somatostatin hormone therapy. The patients were followed for a median of 31 months to assess progression-free survival (PFS) and overall survival (OS). Board-certified specialists in nuclear medicine and radiology analyzed the PET/CT scans, measuring radiotracer uptake in terms of maximum standardized uptake values (SUVmax) in the tumors.
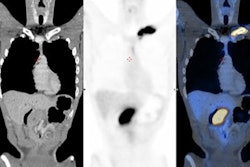
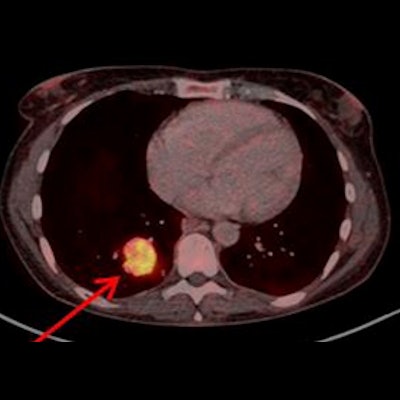
![Example of Ga-68 NODAGA-E[c(RGDyK)]2 PET/CT. Transaxial PET and fused PET/CT and maximum intensity projection with color bars (unit: SUV). Patient with lung neuroendocrine tumor grade 2 (Ki67 15%) with liver and bone metastases. Arrow at primary tumor. Image courtesy of the Journal of Nuclear Medicine.](https://img.auntminnieeurope.com/files/base/smg/all/image/2022/08/ame.2022_08_24_15_29_6786_2022_08_25_NET_tracer.png?auto=format%2Ccompress&fit=max&q=70&w=400) Example of Ga-68 NODAGA-E[c(RGDyK)]2 PET/CT. Transaxial PET and fused PET/CT and maximum intensity projection with color bars (unit: SUV). Patient with lung neuroendocrine tumor grade 2 (Ki67 15%) with liver and bone metastases. Arrow at primary tumor. Image courtesy of the Journal of Nuclear Medicine.
Example of Ga-68 NODAGA-E[c(RGDyK)]2 PET/CT. Transaxial PET and fused PET/CT and maximum intensity projection with color bars (unit: SUV). Patient with lung neuroendocrine tumor grade 2 (Ki67 15%) with liver and bone metastases. Arrow at primary tumor. Image courtesy of the Journal of Nuclear Medicine.The researchers found a significant association between radiotracer uptake and both progression-free survival and overall survival. Patients with an SUVmax above 5.25 had a hazard ratio (HR) of 2.11 for PFS and 6.95 for OS, the researchers wrote.
In addition, with an SUVmax above 5.25, patients experienced a median PFS of 15.5 months, compared with a PFS of 34.3 months below this cutoff level.
"Using Ga-68 NODAGA-E[c(RGDyK)]2 for integrin avb3 PET imaging, integrin avb3 was evident in tumor lesions from both patients with low and high-grade tumors. High tumor uptake of Ga-68 NODAGA-E[c(RGDyK)]2 was associated with a poorer prognosis in regards to both disease progression and death," the authors wrote.
Ultimately, integrin avb3 is a known prognostic marker in NETs and an emerging treatment target, Carlsen and colleagues wrote. They noted that this was a phase II trial, but that Ga-68 NODAGA-E[c(RGDyK)]2 PET/CT so far appears promising as a tool to help develop those treatments.
"Further studies are warranted to establish if Ga-68 NODAGA-E[c(RGDyK)]2 PET/CT may become a tool for risk stratification and for identification of patients eligible for treatments targeting integrin avb3," they concluded.