Radiopharmaceutical developer Nuclidium and the University Hospital Basel, Switzerland, have received an Investigator Award from the Neuroendocrine Tumors Research Foundation to support a phase I trial of its TraceNET copper-based diagnostic radiopharmaceutical for neuroendocrine tumors (NETs).
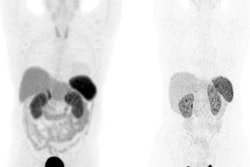
The PET imaging candidate includes a copper-61 (Cu-61) imaging radioisotope and a somatostatin receptor (SSTR) antagonist-targeting molecule that binds to SSTRs overexpressed on the surface of NET cells. It will serve as the diagnostic component of the firm's theranostic program for NET patients.
Expected to begin in the first half of 2023, the phase I clinical trial will assess the safety, biodistribution, pharmacokinetics, and dosimetry of TraceNET in NET patients. It will also compare the performance of TraceNET with Ga-68 DOTATOC on PET/CT imaging for detecting NET in the same patients, according to Nuclidium, whose medical advisor and co-founder is radiologist and nuclear medicine specialist Prof. Dr. Gustav von Schulthess, PhD.
Under Nuclidium's copper-based platform, each product candidate can be adjusted from a diagnostic to a therapeutic by exchanging Cu-61 with Cu-67, according to the company. Both are distributed identically in the body, enabling exact dosimetry for each patient, Nuclidium said.