Higher image quality, lower patient and staff radiation exposure, simultaneous multiorgan imaging, and expanded range for optimal dose imaging are among the significant advantages that total-body PET (TB-PET) can bring to the clinical setting, according to a leading hybrid imaging expert.
How will TB-PET revolutionize routine imaging, and what are its future applications? Prof. Thomas Beyer, PhD, MBA, addressed these and other questions at the recent Conference on Hybrid Imaging Live (CHILI), organized by the European Society for Hybrid, Molecular, and Translational Imaging (ESHIMT) and held virtually.
PET is a molecular, functional imaging modality that gives 4D data with high volume sensitivity, providing the means for very innovative research especially in noninvasive assessment of pathophysiology, noted Beyer, who is the immediate past president of ESHIMT and professor of physics of medical imaging at Medical University of Vienna.
Originally positioned as a means for noninvasive molecular phenotyping and quantification in the 1980s, PET's improved technology in the 2000s generated renewed interest in quantification, which has grown over the last five years.
"Total-body PET pushes optimized imaging protocols and optimized dose imaging, whereby you can easily tweak injected dose, scan time, image quality or extend or defer the time point of the imaging, depending on patient needs," Beyer said in his keynote lecture, titled "New horizons for hybrid imaging -- the next big leap," on 13 November.
 Traditional PET systems have limited range and sensitivity, and high dose exposure. FOV = field-of-view. All images courtesy of Prof. Thomas Beyer, PhD, MBA.
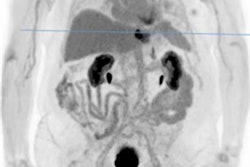
Traditional PET systems have limited range and sensitivity, and high dose exposure. FOV = field-of-view. All images courtesy of Prof. Thomas Beyer, PhD, MBA.Traditionally, PET imaging systems provide an axial field-of-view coverage of about 15-25 cm, sufficient to picture a single organ such as the brain or the heart. Whole-body scans using step-and-shoot, multibed positions, or continuous table motion, and applied in the majority of oncology indications, take 10 to 20 minutes. The exposure associated with the standard injection of FDG for a whole-body scan is about 7 mSv, which is around twice the annual background exposure. Furthermore, lesions smaller than 4 mm to 6 mm are difficult to detect. Importantly, traditional PET performance is relatively poor for late-time-point imaging, essential for antibody-labeled imaging for immune response assessment, Beyer noted.
Extended field-of-view
Standard, clinical PET technology with a fixed ring of detectors detects only about 1% of the photons, he continued. With more detectors (560,000 vs 35,000) in an extended axial field-of-view of up to 200 cm, sensitivity increases from 1% to around 40% for the same amount of injected activity. This increased sensitivity helps to generate protocols that are more amenable to patient throughput scenarios.
"If we extend the axial field-of-view and we are ready to pay for it, can we shorten the scan time to less than one minute? Can we reduce patient exposure to less than 1 mSv? Does it help increase lesion detectability? And can we expand the imaging range after a given injection of a radiotracer, to perform late time point imaging?" Beyer put to attendees.
 Extended field-of-view systems increase sensitivity 40-fold for same injected activity.
Extended field-of-view systems increase sensitivity 40-fold for same injected activity.The latter is important because the longer the uptake time, the better benign from malignant disease and even low-grade from high-grade tissues can be differentiated in a multitude of tumors. Such late-time-point imaging can now be explored with the new extended axial field-of-view imaging PET systems that have entered the market, he noted.
Bright future
TB-PET could represent a quantum leap in future diagnostic or precision medicine for several reasons, Beyer outlined: First, because it could optimize operations. With a higher sensitivity examination time is shortened without a loss in imaging quality. However, increasing the number of patients scanned and thus revenue would also mean spending money on employing more technologists and medical experts to cope with patient throughput and scan interpretation. This 'curse of dimensionality' would also imply structural changes such as the number of uptake rooms and injection machines.
 The curse of dimensionality means organizational changes for higher patient throughput.
The curse of dimensionality means organizational changes for higher patient throughput.That said, novel applications for multiparametric hybrid imaging could more than compensate, Beyer noted. He pointed to one interesting suggestion that envisaged total-body dynamic PET in maternal-fetal medicine, using its advantage of high sensitivity for low injected activity values -- potentially as low as 25 MBq -- to explore fetal development:
Low levels of imaging biomarkers labeled with O15 or C11 to probe oxygen uptake in the fetal brain or nutrient supply of the fetus using amino acids could provide helpful data with less than 0.5 mSv exposure, thereby addressing the issue of stillborn deaths or premature births, a major challenge of healthcare systems across the world, he said.
Higher sensitivity also facilitates ultra low contrast imaging for detecting lesions of 2.5 mm in size in the thorax. This could be leveraged for example to assess the presence of micrometastases, or low levels of inflammation, and to evaluate tumor stroma. Further, for late time point imaging, isotopes such as Zirconium that have a 3.3-day half-life can be used for optimal (Zr-labeled) antibody imaging, a prerequisite for assessing tumor response and immunotherapy.
Other higher sensitivity benefits included reducing phase I drug trials and their costs via microdosing human subjects, as well as the capacity for interorgan signaling to describe diseases.
"Today we see many more promises of extended field-of-view PET, than proven benefits. In order to make total-body a quantum leap truthfully, we have to all engage in turning these promises into benefits," Beyer concluded.