Permanent seed implant brachytherapy, in which a small radiation source is implanted in or close to a tumor, is a common treatment for early stage prostate or breast cancer. The seeds are based on radioisotopes such as I-125 and Pd-103. But the low-energy photons emitted by such sources create significant challenges for treatment planning because absorbed dose is strongly affected by tissue atomic composition at low energies. Now, a research team from Canada and the Netherlands has developed a brachytherapy dosimetry method that accounts for dose heterogeneity.
Currently, brachytherapy dose distributions are determined using the AAPM TG-43 formalism, which calculates dose in water and ignores tissue heterogeneities. The researchers -- from the University of Toronto and Erasmus University in Rotterdam -- have proposed a scheme that can calculate absorbed dose in heterogeneous tissue surrounding a brachytherapy seed, by multiplying the TG-43-calculated dose in water by an inhomogeneity correction factor (ICF).
The ICF method requires knowledge of the tissue's bulk properties, such as the attenuation coefficient and mass energy absorption coefficient. In their latest work, the researchers demonstrate the use of dual-energy CT (DECT) imaging to extract these parameters (Physics in Medicine and Biology, 22 August 2014, Vol. 59:18, pp. 5305-5316).
"We previously reported a correction factor, the ICF, for the TG-43 to include heterogeneity corrections in seed brachytherapy dosimetry," explained lead author Dr. Jean-Philippe Pignol, PhD, a scientist at the Sunnybrook Research Institute in Toronto. "To accurately calculate the ICF, we need to accurately quantify subtle difference in tissue composition, and this is enabled using dual-energy CT."
Phantom tests
To assess their proposed approach, Pignol and colleagues obtained DECT images of a heterogeneous phantom using a commercial CT scanner. The phantom is composed of two acrylic cylinders, each containing four inserts of materials mimicking muscle (acrylic), fat (polypropylene), water (Virtual Water), and bone (Teflon).
Performing the CT scans at two energies (140 and 90 kVp) enables calculation of the attenuation and mass energy absorption coefficients needed for the ICF formulation. The ICF calculation also requires imaging of two reference materials -- cylinders of water and polyethylene in this case, which were placed by the phantom and scanned concurrently.
The researchers loaded each pair of phantom inserts with a Pd-103 seed and calculated the resulting dose distributions using the TG-43 and ICF-corrected TG-43 formalisms. Results were compared with measurements using Gafchromic film placed between the phantom's two cylinders. They assessed the accuracy of the calculated dose distributions by calculating the gamma index (GI) passing rate for 2%/2 mm criteria.
 Isodose lines for the case of seeds loaded in the pair of acrylic inserts. Blue, green, and red contours represent film dosimetry measurements, TG-43 and ICF-corrected TG-43 dose distributions, respectively.
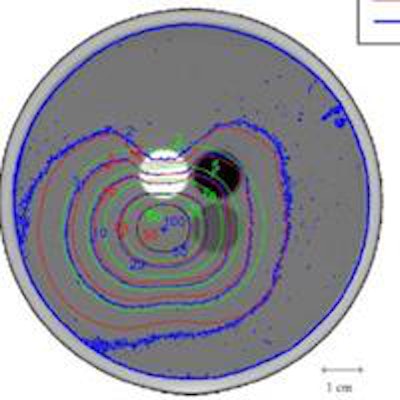
Isodose lines for the case of seeds loaded in the pair of acrylic inserts. Blue, green, and red contours represent film dosimetry measurements, TG-43 and ICF-corrected TG-43 dose distributions, respectively.Dose distributions generated using the ICF formalism showed better agreement with film measurements than the TG-43 distributions did. For seeds inserted in acrylic, for example, the isodose lines for film and ICF calculations bent in toward the Teflon rod (due to its higher attenuation compared with water) and bulged out from the polypropylene rod (which attenuates less than water). ICF-calculated isodose lines matched the measurements well, while the TG-43 dose distribution was unaffected by the heterogeneities. The GI passing rate for 2%/2 mm was 22.0% for TG-43, improving to 75.8% for ICF dose distributions.
For seeds inserted in the Teflon rods, the GI passing rate was 15.2% for TG-43 and 69.2% for the ICF formalism. For seeds in polypropylene inserts, the TG-43 formalism underestimated dose everywhere but in Teflon, with a GI passing rate of 28.6%, increasing to 77.2% with the ICF. For seeds loaded in Virtual Water, the GI passing rate improved from 21.2% for TG-43 to 71.9% with the ICF.
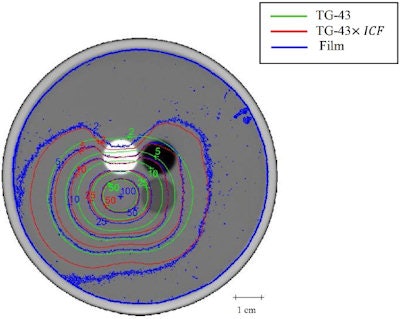
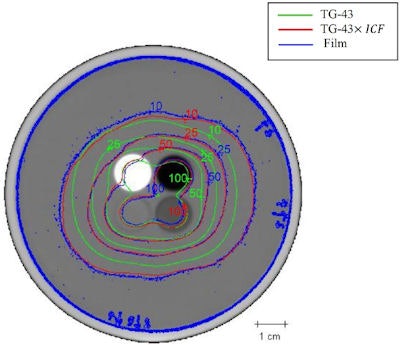 Isodose lines for seeds placed in all inserts. Blue, green, and red contours represent film dosimetry measurements, TG-43 and ICF-corrected TG-43 dose distributions, respectively.
Isodose lines for seeds placed in all inserts. Blue, green, and red contours represent film dosimetry measurements, TG-43 and ICF-corrected TG-43 dose distributions, respectively.Finally, the researchers examined the dose distributions with seeds placed in all of the inserts -- creating a seed implant configuration of eight seeds spaced 1.5 cm apart in a cubic structure. Here, the GI passing rate improved from 40.8% to 90.5% when ICF was added to TG-43. This excellent agreement with film measurements -- attributed to smoothing effects and cross coverage of other seeds -- is particularly important because clinical implants employ multiple seeds to deliver dose.
The researchers note their ICF methodology could be relatively easily integrated into a clinical setting, as standard TG-43 seed parameters are already published and well defined. Furthermore, the use of DECT rather than conventional CT enables direct extraction of the required tissue parameters from CT images, eliminating the need for tissue segmentation and removing the inherent errors associated with assigning population-based atomic compositions.
In the future, Pignol said, the ICF will be implemented into a new version of a commercial planning system that will be available to the community. "We are currently reviewing previous plans and found that the ICF seems to better predict for toxicity," he said. "We will publish those results soon, along with the recommendation that ICF should become standard."
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.