Gold nanoparticles (GNPs) make ideal contrast agents for identifying tumors. They can be attached to antibodies to actively target specific tumor characteristics, while leaky vasculature and poor lymphatic drainage around tumor sites leads to increased nanoparticle uptake in tumor cells. However, there are currently no imaging systems with high enough detection sensitivity to GNPs at sufficient tissue depth for in vivo and in vitro studies.
To address this limitation, researchers from University College London (UCL) in the U.K. and National Institute of Nuclear Physics (INFN) in Italy have developed a new GNP imaging method that works by detecting the L-edge x-ray fluorescence (L-XRF) emitted when gold is irradiated with 15 keV x-rays. The technique can achieve increased detection sensitivity at greater depths than current optical modalities (Physics in Medicine and Biology, 7 November 2013, Vol. 58:21, pp. 7841-7855).
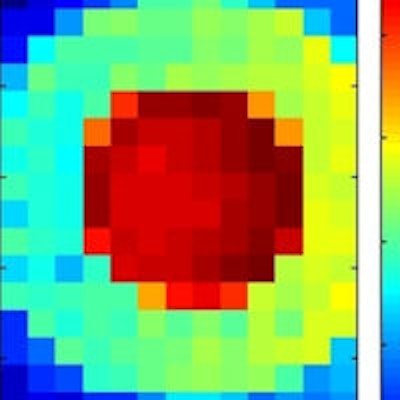
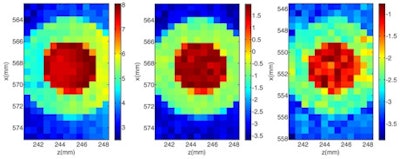 XRF images (log scale) of GNP contrast phantoms acquired using an SDD coupled to a focusing polycapillary optic. The primary beam is incident on the right-hand side of the images. Left: High-contrast phantom; middle: Scatter normalized high-contrast phantom; right: Coherent scatter normalized low-contrast phantom. All images courtesy of Kate Ricketts.
XRF images (log scale) of GNP contrast phantoms acquired using an SDD coupled to a focusing polycapillary optic. The primary beam is incident on the right-hand side of the images. Left: High-contrast phantom; middle: Scatter normalized high-contrast phantom; right: Coherent scatter normalized low-contrast phantom. All images courtesy of Kate Ricketts.The INFN group designed two L-XRF imaging systems: a step-and-scan spectroscopic module and an energy-resolving pixelated detector. The step-and-scan system consisted of a silicon drift detector (SDD) coupled to a focusing polycapillary optic (a bundle of hollow glass capillaries) that directs the emitted x-rays to the detector chip.
The second system was designed to avoid slow step-and-scan acquisition, by exciting the whole sample with a wide beam and detecting the fluorescent x-rays with a pixelated energy-resolving detector. For this "one-shot" approach, images were formed using a controlled drift detector (CDD) coupled to a collimating polycapillary optic, which provided the position at which the fluorescence was emitted.
"We implemented the SDD system to demonstrate the sensitivity of such high-energy-resolution technology to low GNP concentrations. We extended the single-channel detector to a multiple-channel pixelated CDD system to demonstrate the use of an array of energy-resolving pixels for GNP x-ray fluorescence imaging in a single shot, and to demonstrate the spatial resolution improvements that are possible," explained Kate Ricketts, a research associate in the department of medical physics and bioengineering at UCL. "We are currently developing a greater energy-resolving pixelated detector to marry the benefits of both systems into one imaging device."
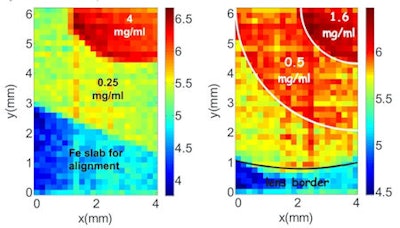 XRF images (log scale) of GNP contrast resolution phantoms using the CDD-polycapillary optic XRF system. The images are of high-contrast (left) and low-contrast (right) details.
XRF images (log scale) of GNP contrast resolution phantoms using the CDD-polycapillary optic XRF system. The images are of high-contrast (left) and low-contrast (right) details.Phantom studies
Ricketts and colleagues used both systems to image two GNP phantoms with concentric rings containing different concentrations of GNPs. The high-contrast phantom contained 4 mgAu/ml and 0.2 mgAu/ml GNP solutions, while the low-contrast phantom contained 0.125 mgAu/ml and 0.025 mgAu/ml solutions.
Step-and-scan imaging was performed at the U.K.'s Diamond Synchrotron Source. The sample was scanned with a 600 x 600 µm pitch to build up a pixelated 12 x 7.2 mm image. The acquisition time was 40 seconds per point, with a total imaging time of 3 hours. The researchers reconstructed two-dimensional GNP images by computing the integral counts under the gold La and Lb peaks. For both phantoms, the regions of different concentrations were clearly defined. Implementing a scatter normalization technique to account for beam absorption improved the image uniformity.
They tested the one-shot system at the Elettra synchrotron source in Italy. Again, XRF images of the high- and low-contrast phantoms clearly showed regions of differing concentration. This CDD/parallel optic system improved the spatial resolution by a factor of 1.6 compared with the SDD/focusing optic system. It also had an acquisition time nearly three orders of magnitude less than the step-and-scan imager.
Both systems could distinguish GNP concentrations differing by factors of twenty and five, thereby achieving the requirement needed to distinguish tumor from healthy tissue at GNP concentrations relevant for in vivo and in vitro studies (20:1 and 5:1 contrast using functionalized and unfunctionalized GNPs, respectively).
','dvPres', 'clsTopBtn', 'true' );" >
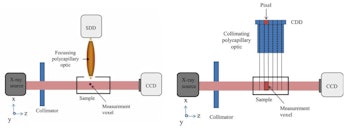
Click image to enlarge.
Experimental setup for 2D (left) and CDD XRF (right) imaging systems. The CCD cameras behind the samples monitor the beam-sample alignment.
Depth determination
To investigate the effect of overlying tissue on the XRF signal, Ricketts and colleagues used the step-and-scan L-XRF system to image an 8 mgAu/ml GNP solution with five thicknesses of Perspex (0, 2, 4, 8, and 16 mm) placed between the sample and detector. Each measurement was acquired for 180 seconds.
The gold L-XRF lines decreased in intensity with increasing Perspex thickness, but all signals remained above the system's detection limit. The measured signal reductions due to overlying thicknesses of Perspex agreed well with predictions. For example, the reductions in signal due to 8 mm of Perspex were measured as 84% and 95% for the gold Lb and La signals, respectively, compared with predicted values of 92% and 97%.
The researchers suggest that even though the Lb signal has a lower fluorescence yield than the La peak, it may be beneficial to use the Lb peak for measurements at depth as it has a lower attenuation coefficient. They conclude that the penetrability of L-XRF is sufficient for imaging GNP distribution in near-surface small-animal studies and for imaging 3D in vitro cellular constructs.
The team is now developing biomimetic 3D cellular phantoms incorporating cancer cell masses that have taken up nanoparticles at typical in vivo concentrations. "The purpose of the 3D biomimetic cancer models is to provide a controllable nanoparticle uptake imaging platform whereby we can engineer nanoparticle concentrations and distributions over a range of cell types and densities," Ricketts told medicalphysicsweb. "We will then use our imaging system to monitor uptake of nanoparticles in cells, under 3D in vitro conditions, to investigate mechanisms of uptake and the effect of cellular and environmental conditions on targeted nanoparticle uptake."
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.