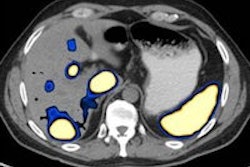
Heavy ion therapy produces high-linear-energy-transfer (LET) Bragg peaks that offer one way to tackle hypoxic -- or oxygen-deficient -- tumors that respond poorly to radiotherapy. However, if delivered across an entire tumor, high LET can also result in the excessive exposure of adjacent healthy tissue and unacceptable side-effects.
Instead, researchers in Denmark and Germany are investigating the targeted approach of LET-painting, where high-LET radiation is steered into the hypoxic portion of a tumor only. In an initial modeling study, they predict superior tumor control compared to the current clinical standard of nonpainted treatments that assume normoxic -- or oxygenated -- tumor composition (Acta Oncologica, 10 September 2013).
 From left to right: Lise Saksø Mortensen, Brita Singers Sørensen, Jakob Toftegaard, Jørgen Petersen, and Niels Bassler. Image courtesy of Michael Harder.
From left to right: Lise Saksø Mortensen, Brita Singers Sørensen, Jakob Toftegaard, Jørgen Petersen, and Niels Bassler. Image courtesy of Michael Harder."LET-painting could quite dramatically increase tumor control, without increasing the side effects that are normally associated with high-LET radiation," said Niels Bassler, PhD, first author and physicist at Aarhus University in Denmark.
The researchers compared the tumor control probabilities (TCP) calculated for LET-painted and nonpainted carbon-12 and oxygen-16 treatment plans in a patient already treated for oropharyngeal cancer. Nonpainted plans mimicked existing clinical approaches, using two coincident pairs of opposed, scanned beams, each delivering a spread out Bragg peak (SOBP) that gave a uniform dose and largely uniform dose-averaged LET across the entire tumor volume. TCPs for these plans were calculated for two scenarios: the first assumed a normoxic tumor, as is the current practice in centers delivering carbon-12 treatments, while the second took tumor hypoxia and the associated radioresistance into account.
Ramped fields steer LET
Painted plans were calculated for a range of hypoxic volume sizes up to 10 cm3, centered on a hypoxic volume identified in the patient's tumor that was delineated using F-18 fluoroazomycin arabinoside (FAZA) PET imaging. The same field arrangement was used as in the nonpainted plans, but the parts of each field incident on the hypoxic volume were ramped, the SOBPs delivering 100% of the dose at the proximal edge of the volume and 0% dose at the distal edge.
The opposed ramped fields elevated dose-averaged LET across the hypoxic volume compared with the surrounding nonhypoxic tumor. Total energy fluence was fixed across all the plans, pinning it to the value that gave a TCP of 95% in the clinical planning standard of the nonpainted plans that assumed a normoxic tumor. This ensured fair comparisons of the painted and nonpainted approaches.
','dvPres', 'clsTopBtn', 'true' );" >
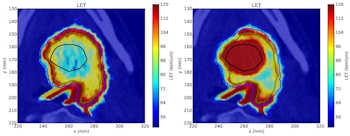
Click image to enlarge.
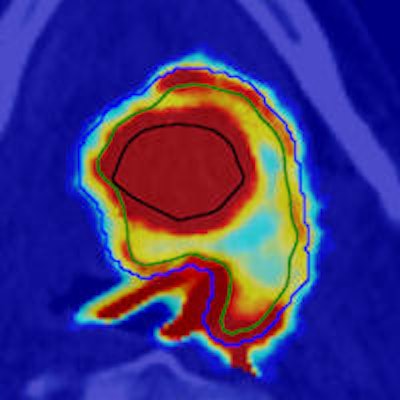
The nonpainted oxygen-16 plan (left) has elevated dose-averaged LET around the edge of the tumor, produced by the distal portion of the Bragg peak while the painted plan (right) also has elevated dose-averaged LET across the hypoxic volume, due to the ramped treatment fields. Image courtesy of Jakob Toftegaard.
LET-painting proved feasible, demonstrating consistently superior TCP values to the clinical reality of a nonpainted plan delivered to a hypoxic tumor; this returned a TCP of 0%. However, this advantage dropped off sharply as hypoxic volume increased, with TCPs of the order of 50% or more only observed in volumes less than 1 cm3 in the oxygen-16 plans and less than 0.5 cm3 in the carbon-12 plans.
Volume dilutes LET
The trend arises from the differences in SOBP composition required to cover the range of hypoxic volumes. As the width of the hypoxic volume increases, so too does the energy range of the SOBP needed to ensure dose coverage. At a given depth, this translates to increasingly large contributions to the dose-averaged LET from the plateaus of the individual Bragg curves. These exhibit significantly lower LET than the peaks of the curves.
To negate the volume effects, the researchers boosted dose to the hypoxic volume in the oxygen-16 plans, while still limiting the total energy fluence used to irradiate the tumor. A boost of 5% produced a TCP of around 50% for a 2 cm3 hypoxic volume, around double that achieved for the nonboosted case.
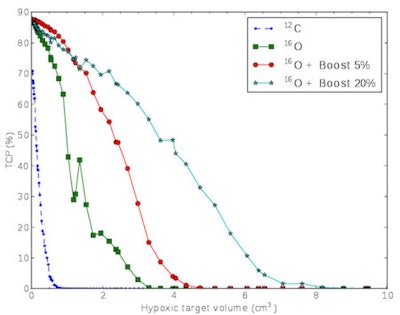 Tumor control drops off as the painted hypoxic volume increases in size. Oxygen-16 ion beams are associated with higher LET, allowing larger hypoxic volumes to be controlled, particularly when accompanied by a small dose boost. Image courtesy of Jakob Toftegaard.
Tumor control drops off as the painted hypoxic volume increases in size. Oxygen-16 ion beams are associated with higher LET, allowing larger hypoxic volumes to be controlled, particularly when accompanied by a small dose boost. Image courtesy of Jakob Toftegaard.Overall, carbon-12 ions resulted in significantly lower tumor control than the oxygen-16 ions. "We might need ions heavier than carbon-12 ions to achieve significant increases in tumor control," Bassler said. "Scanning beams of oxygen-16 ions, which are available at the heavy ion facility in Heidelberg, seem to be a good candidate here."
He added that while the results are encouraging, much more research is needed before LET-painting can be introduced clinically, including similar studies with other radiobiological models and extensive in vitro and in vivo testing.
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.