A deep-learning algorithm that incorporates information from prior low-dose CT (LDCT) exams is more effective for estimating three-year malignancy risk of pulmonary nodules than models that use a single CT exam alone, Dutch researchers have found.
The study results underscore how adding AI to imaging data can help radiologists better characterize pulmonary nodules found on LDCT -- a task that can be difficult, according to a team led by Kiran Venkadesh of Radboud University Medical Center in Nijmegen, the Netherlands. The group's research was published on 1 August in Radiology.
"[It] is challenging for radiologists to identify and monitor potentially malignant nodules," the group wrote. "Despite the presence of nodule management guidelines, accurate characterization remains tedious and is subject to inter- and intrareader variability ... Artificial intelligence using deep learning has demonstrated promising results for accurately estimating the malignancy risk of pulmonary nodules, especially compared with histopathologic analysis-based reference standards."
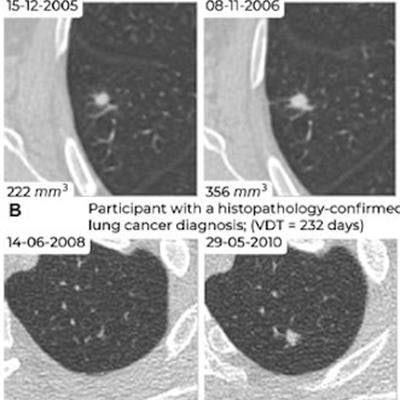
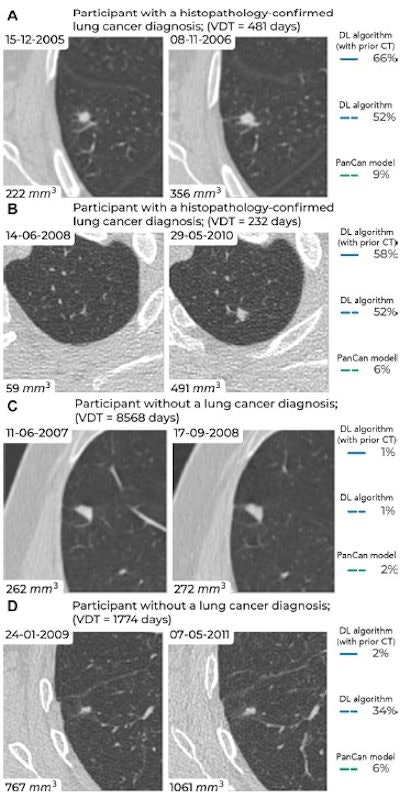 Examples of screening-detected pulmonary nodules from the Danish Lung Cancer Screening Trial (DLCST) and the Multicentric Italian Lung Detection Trial (MILD), wherein malignancy risks were estimated accurately by the deep-learning (DL) algorithm that combines a current and prior CT examination. The lines correspond to the malignancy risk estimation algorithms (solid blue, DL algorithm with prior CT; dotted blue, DL algorithm; dotted green, PanCan model). The percentages correspond to the risk scores from 0% to 100%. (A) Annual low-dose axial chest CT images in a 55-year-old woman with a lung cancer diagnosis in the DLCST show a growing spiculated malignant nodule, with a volume doubling time (VDT) of 481 days. All algorithms produced high malignancy risk scores. (B) Biennial low-dose axial chest CT images in a 67-year-old man with a lung cancer diagnosis in the MILD show a growing malignant nodule (VDT, 232 days). The DL algorithms produced high malignancy risk scores. (C) Annual low-dose axial chest CT images in a 66-year-old male participant without a lung cancer diagnosis in the DLCST show a stable benign nodule in which all algorithms produced low malignancy risk scores. (D) Biennial low-dose axial chest CT images in a 78-year-old male participant without a lung cancer diagnosis in the MILD show a stable part-solid benign nodule, in which the DL algorithm, which combines current and prior CT, produced a low malignancy risk score. However, the algorithm that only processed a single CT produced a high malignancy risk score. Images and caption courtesy of the RSNA.
Examples of screening-detected pulmonary nodules from the Danish Lung Cancer Screening Trial (DLCST) and the Multicentric Italian Lung Detection Trial (MILD), wherein malignancy risks were estimated accurately by the deep-learning (DL) algorithm that combines a current and prior CT examination. The lines correspond to the malignancy risk estimation algorithms (solid blue, DL algorithm with prior CT; dotted blue, DL algorithm; dotted green, PanCan model). The percentages correspond to the risk scores from 0% to 100%. (A) Annual low-dose axial chest CT images in a 55-year-old woman with a lung cancer diagnosis in the DLCST show a growing spiculated malignant nodule, with a volume doubling time (VDT) of 481 days. All algorithms produced high malignancy risk scores. (B) Biennial low-dose axial chest CT images in a 67-year-old man with a lung cancer diagnosis in the MILD show a growing malignant nodule (VDT, 232 days). The DL algorithms produced high malignancy risk scores. (C) Annual low-dose axial chest CT images in a 66-year-old male participant without a lung cancer diagnosis in the DLCST show a stable benign nodule in which all algorithms produced low malignancy risk scores. (D) Biennial low-dose axial chest CT images in a 78-year-old male participant without a lung cancer diagnosis in the MILD show a stable part-solid benign nodule, in which the DL algorithm, which combines current and prior CT, produced a low malignancy risk score. However, the algorithm that only processed a single CT produced a high malignancy risk score. Images and caption courtesy of the RSNA.Lung cancer causes the most cancer-related fatalities around the world, and early diagnosis through regular LDCT screening is key to improving patient outcomes, the team explained. But LDCT can flag lung nodules that are benign, making it important to develop tools that can help clinicians better characterize them. AI algorithms show promise in this regard, according to Venkadesh and colleagues.
The investigators evaluated the performance of a deep-learning algorithm that incorporates prior LDCT exam information, comparing this combination to a deep-learning algorithm plus a single LDCT exam and the Pan-Canadian Early Lung Cancer Detection Study (PanCan) model (PanCan is a study that uses a risk-prediction algorithm to assess lung cancer screening's cost-effectiveness).
The deep-learning algorithm's training set consisted of 10,508 nodules (422 of which were malignant, or 4%) in 4,902 trial participants; training set data came from the National Lung Screening Trial (NLST). Two test sets consisted of 129 nodules (43 of which were malignant, or 33%) and 126 nodules (42 of which were malignant, also 33%); these data came from the Danish Lung Cancer Screening Trial (DLCST) and the Multicentric Italian Lung Detection (MILD) Trial.
The deep-learning algorithm that incorporated previous LDCT exam data outperformed both the algorithm plus a single LDCT exam and the PanCan model, the team reported.
| Area under the curve (AUC) of deep-learning algorithm for predicting pulmonary nodule malignancy (with 1 as reference) | |||
| External test set | PanCan model | Deep-learning algorithm with single LDCT exam | Deep-learning algorithm plus prior LDCT exam data |
| 1 | 0.64 | 0.85 | 0.91 |
| 2 | 0.63 | 0.89 | 0.94 |
The study makes a valuable contribution to the current literature, wrote Carolyn Horst, PhD, of King's College London in the U.K. and colleague Dr. Mizuki Nishino of Brigham and Women's Cancer Center in Boston in an accompanying commentary.
"The research published by Venkadesh et al is the first step in using artificial intelligence in longitudinal imaging within the lung cancer screening sphere, paving the way for the integration of artificial intelligence and serial imaging to achieve improved outcomes for participants and screening programs alike," they wrote.
The complete study can be found here.