Better characterization of disease progression gained through quantifying microstructure features with diffusion MRI can help lead to an earlier diagnosis, more timely access to treatment, and the development of targeted therapies for neurological and psychiatric disorders, according to award-winning Swiss researchers.
Quentin Uhl and his colleagues from the department of radiology at Lausanne University Hospital (CHUV) have developed a neurite exchange imaging (NEXI) model designed to quantify microstructure features of the human cortex in vivo. The development has significant implications for general radiologists, especially neuroradiologists, Uhl told AuntMinnieEurope.com.
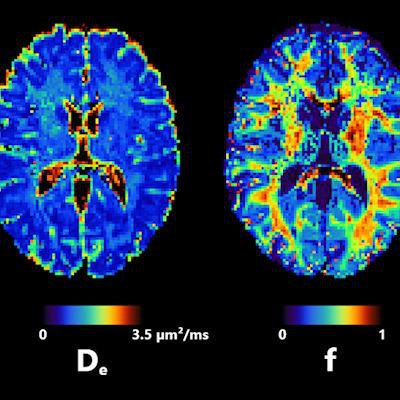
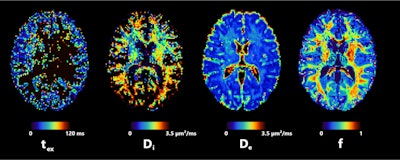 Axial slice of NEXI parametric maps in one subject. The first and third images are consistent throughout the cortex; the first image seems to have more white matter, but this cannot be reliably estimated using available diffusion times. The fourth image displays the expected anatomical pattern in white versus gray matter. The second image shows large variability across voxels while hitting its upper bound frequently. All figures courtesy of Quentin Uhl et al and presented at ISMRM 2023.
Axial slice of NEXI parametric maps in one subject. The first and third images are consistent throughout the cortex; the first image seems to have more white matter, but this cannot be reliably estimated using available diffusion times. The fourth image displays the expected anatomical pattern in white versus gray matter. The second image shows large variability across voxels while hitting its upper bound frequently. All figures courtesy of Quentin Uhl et al and presented at ISMRM 2023."By quantifying features in the cortex such as neurite density or cell membrane permeability and how they change in pathology, the NEXI model has the potential to enhance our understanding and management of neurological and psychiatric diseases," he said.
"NEXI is particularly advantageous, thanks to its implementation on clinical scanners. This enables studies in large cohorts comprising both healthy individuals and patients, facilitating extensive research on the subject," Uhl continued. "The model provides valuable information regarding water exchange between neurites and the extracellular space. This information can be used to derive neurite permeability, serving as a proxy for assessing neurite health/condition within each voxel."
Award-winning study
At the 2023 International Society for Magnetic Resonance in Medicine (ISMRM) annual meeting, the CHUV group received a summa laude award for its work on quantifying human gray matter microstructure using NEXI and 300 mT/m gradients.
Working with the Cardiff University Brain Research Imaging Centre in the U.K., the team used a gray matter two-compartment diffusion model that accounts for intercompartment exchange. The model has first been implemented in a preclinical setting in vivo and ex vivo.
 Quentin Uhl and his prizewinning poster at ISMRM 2023.
Quentin Uhl and his prizewinning poster at ISMRM 2023.The researchers tested the performance of NEXI in the human cortex in vivo, using the Connectom strong gradient system to achieve short diffusion times and high b-values. They scanned four healthy volunteers, three of whom were rescanned on a different day. An MPRAGE sequence was acquired for anatomical reference (1-mm isotropic resolution). Diffusion-weighted images were obtained on a 3-tesla system, and the data for the study came from the U.K. National Facility for In Vivo MR Imaging of Human Tissue Microstructure.
"Importantly, NEXI estimates displayed good scan-rescan reproducibility while retaining sensitivity to inter-subject differences," the authors wrote.
What lies ahead?
Future efforts will focus on possible improvements, such as more advanced acquisition schemes.
"The question that remains to be clarified is whether we can propose a more elaborate model that quantifies cell bodies in addition to neurites and membrane permeability while still compatible with a clinical diffusion MRI acquisition," stated Uhl, who is a PhD candidate at the Lemanic Neuroscience Doctoral School in Lausanne.
One potential avenue for the future would be to develop a machine-learning model to speed up the estimation of the NEXI parametric maps but also, using explainable AI concepts, to select the optimal acquisition protocol for NEXI estimation and thus minimize scan time, he explained.
The co-authors of the ISMRM e-poster were Tommaso Pavan, Malwina Molendowska, Derek K. Jones, Marco Palombo, and Ileana Jelescu.