The U.K. Royal College of Radiologists (RCR) has published a 40-page document in a bid to boost uniformity and consistency in the treatment of head and neck cancer.
"The aim is to reduce unacceptable variation in U.K. radiotherapy," explained Dr. Amen Sibtain, chair of the RCR working group that produced the new report. "These head and neck consensus statements follow from excellent work done by the RCR in the areas of breast and lung cancer."
The document will serve as a practical stimulus for head and neck cancer teams to review their current radiotherapy service to ensure they can deliver optimal treatment for their patients, noted Sibtain, who is the clinical lead in head and neck cancer at St Bartholomew's Hospital in London and an oncology advisor with BBC TV Drama.
"The RCR's consensus statements are produced to guide and support clinicians in controversial areas of practice that lack strong evidence," he added.
The following six main areas are covered in the report:
- Gross tumor volume (GTV) to clinical target volumes (CTV) margins
- Unilateral radiotherapy to the oropharynx
- Reducing the CTV to improving organ sparing
- Adjuvant contralateral neck irradiation following surgery for oral tongue cancer
- Induction chemotherapy
- Radical reirradiation in head and neck cancer
While clinical consensus statements and clinical guidelines both provide recommendations on clinical practice, there are subtle but important differences between them, explained Sibtain, an RCR examiner in oncology and a medical advisor for Cancer Research UK and Macmillan, and his fellow authors.
"Clinical guidelines are usually based on a formal systematic review of high-level evidence, while consensus statements are most appropriate on topics where evidence is limited or lacking and therefore where a consensus approach offers the best way to address variability in clinical practice and improve patient outcomes," they stated.
Focus on CT
One area covered in the document is non-nasopharyngeal head and neck squamous cell carcinoma (HNSCC) patients treated with radical or adjuvant radiotherapy in whom the contralateral high level II (HLII) lymph nodes in an uninvolved contralateral neck were omitted.
HLII is defined as the most cranial axial CT image where the posterior belly of the digastric muscle crosses the jugular vein, ensuring irradiation of the subdigastric lymph node.
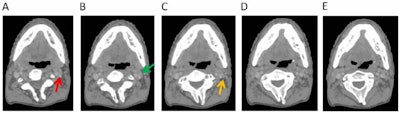 Omission of high level II (HLII) where posterior belly of digastric crosses the posterior aspect of internal jugular vein. Example of axial planning CT slices with intravenous contrast (2 mm slice thickness). A is the most caudal slice; A to E extends superiorly. The level where the posterior belly of digastric muscle crosses posterior aspect of internal jugular vein is shown in C, and it would be the most superior slice of clinical target volume (CTV) delineation, when HLII is omitted from elective CTVs. Red arrow in A: internal jugular vein. Green arrow in B: posterior belly of digastric muscle. Yellow arrow in C: posterior belly of digastric crosses posterior aspect of internal jugular vein. All images courtesy of RCR.
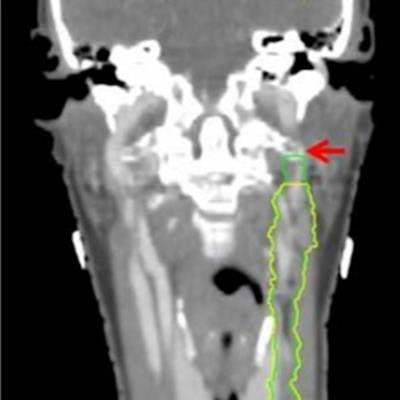
Omission of high level II (HLII) where posterior belly of digastric crosses the posterior aspect of internal jugular vein. Example of axial planning CT slices with intravenous contrast (2 mm slice thickness). A is the most caudal slice; A to E extends superiorly. The level where the posterior belly of digastric muscle crosses posterior aspect of internal jugular vein is shown in C, and it would be the most superior slice of clinical target volume (CTV) delineation, when HLII is omitted from elective CTVs. Red arrow in A: internal jugular vein. Green arrow in B: posterior belly of digastric muscle. Yellow arrow in C: posterior belly of digastric crosses posterior aspect of internal jugular vein. All images courtesy of RCR.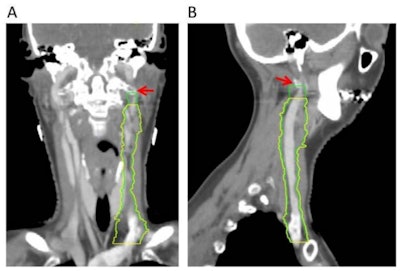 HLII sparing. Coronal (A) and sagittal (B) planning CT images show elective lymph node level contouring of left neck levels II-IVa with (yellow contours) and without (green contours) sparing of HLII. Red arrow: transverse process of C1.
HLII sparing. Coronal (A) and sagittal (B) planning CT images show elective lymph node level contouring of left neck levels II-IVa with (yellow contours) and without (green contours) sparing of HLII. Red arrow: transverse process of C1.The most superior CT slice for level II delineation when HLII is spared has been defined in the ongoing trial of proton beam radiotherapy for oropharyngeal cancer (TORPEdO) to be where the posterior belly of digastric crosses the posterior aspect of the internal jugular vein, the authors pointed out. Also important here is work by clinical oncologist Dr. Zsuzsanna Iyizoba Ebozue and colleagues at Leeds Cancer Centre (Radiotherapy for oropharyngeal carcinoma with a clinically uninvolved contralateral neck: the safety of omission of contralateral high level II and retropharyngeal lymph nodes from elective target volumes. Clin Oncol 2021 in press).
Consultation process
Putting together the RCR document involved extensive discussions. Head and neck leads and multidisciplinary teams from all U.K. cancer facilities that deliver radiotherapy were invited to contribute to the draft statements with and provide feedback. They also completed a survey on current head and neck radiotherapy practice.
Among the participating groups were the Society and College of Radiographers, Institute of Physics and Engineering in Medicine, Association of Cancer Physicians, the Swallows Head and Neck Cancer Group, and clinical trial leads.
"On 6 July 2021 head and neck leads from each centre were invited to attend a virtual consensus meeting to discuss and vote on the draft statements. Fifty centres were represented," the authors wrote. "Initial discussions were had in small breakout rooms followed by a whole-group discussion facilitated by the working group. Many statements were refined based on the meeting discussions."
The final statements were then approved by the RCR's Clinical Oncology Professional Support and Standards Board for publication.
"These are recommendations for activities or interventions that could be used but where discussion with clinical teams and the patient, carefully considering the alternatives, is advised," the authors stated.
The RCR consensus process was initially developed in 2016 to help reduce variation in U.K. radiotherapy practice. They should be adopted in parallel with relevant National Institute for Health and Care Excellence (NICE) guidance, explained Sibtain and Dr. Tom Roques, medical director for professional practice at the RCR.
To download the guidelines, visit the RCR website.