A clear understanding of the pitfalls in the performance and interpretation of adrenal CT can help prevent incorrect and inappropriate investigations, award-winning researchers from a top London facility have found. It's essential to keep aware of the full range of pseudolesions and mimics, they said.
"Evaluation of adrenal tumor function is limited on imaging, but may be inferred from imaging findings," noted Dr. Gurinder Nandra and colleagues from the department of radiology at St. George's University Hospitals NHS Foundation Trust in an e-poster presentation that received a cum laude award at RSNA 2018 in Chicago.
Other adrenal pathology, including metastases and adrenocortical carcinoma, may be encountered, and this means it's important to know about the imaging approaches to evaluate the adrenals, the authors pointed out.
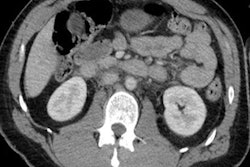
Incidental adrenal nodules are identified in around 5% of patients who undergo CT. The prevalence of detecting incidentalomas increases with age, but most incidentally encountered adrenal pathology is benign and of little clinical relevance, they wrote. Adenomas are by far the most common adrenal pathology identified.
Among the technical aspects that deserve special attention are the following:
- The region of interest (ROI): Changing the size of the ROI can alter the perceived attenuation of the nodule. The ROI should cover at least two-thirds of the circumference of the nodule, and exclude tiny areas of heterogeneity from the ROI (e.g., flecks of calcification) that are not representative of the adrenal pathology. Unenhanced attenuation of less than 10 Hounsfield units (HU) can be used to diagnose lipid-rich adrenal adenoma (sensitivity 71%, specificity of 98%).
- Attenuation values on unenhanced CT: A homogenously dense lesion on unenhanced CT suggests a lack of microscopic lipid content. If attenuation on unenhanced CT is greater than 20 to 30 HU, evaluate the enhancement kinetics with CT.
- Effect of kVp on attenuation values in a dual energy study: To use threshold of less than 10 HU to diagnose a lipid-rich adrenal adenoma, the kVp should be 120. Changing kVp can alter the attenuation values of soft tissues and adrenal glands.
- Timing of post-contrast acquisitions: "Imaging needs to be performed at the correct times to allow sufficient time for enhancement and washout of contrast. Post-contrast images should be obtained at 60 to 75 seconds and 15 minutes," the authors stated.
- Assessment of washout on nondedicated studies: Relative washout can be calculated on nondedicated studies if more than one acquisition is made within 15 minutes post-intravenous contrast.
- Suspicious attenuation: Attenuation of more than 43 HU on noncontrast CT is suspicious for malignancy, regardless of washout characteristics. PET/CT is of more use than CT and MRI in such cases, and adrenal hemorrhage also is a consideration at this attenuation.
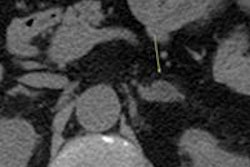
- Evaluation of small nodules: Minor nodularity of less than 1 cm in diameter does not require further radiological investigation. Also, CT evaluation of small adrenal nodules is limited by partial volume artifacts. MRI evaluation of small adrenal nodules is limited by the India ink artifact, or black boundary artifact, on an out-of-phase sequence. This artifact may give the impression of signal loss and lead to an incorrect diagnosis of a lipid-rich adenoma.
- Evaluation of large adrenal masses: Malignancy risk increases with size (over 4 cm, 70%; over 6 cm, 85%) when excluding myelolipoma. In the absence of known malignancy, an adrenal lesion of less than 4 cm with indeterminate imaging features is likely to be benign.
- Enhancement characteristics of metastases: Enhancement/washout characteristics of adrenal metastases are variable, and they can be confused with pheochromocytoma.
- Adrenal calcification: Calcification is seen in benign adrenal pathology, but also can be seen in cases of malignancy, including adrenocortical carcinoma. "Look for ancillary features of malignancy including size, heterogeneity and invasion," the authors recommended. "Evaluation of a predominantly calcified adrenal lesion will be limited with chemical shift MRI."
- Heterogeneous signal loss: Heterogeneous signal loss is not typical for a small lipid-rich adenoma and raises the possibility of malignant pathology. It also can be seen in larger adenomas because of calcification/cystic change/myelolipomatous metaplasia.
In their RSNA 2018 exhibit, Nandra and colleagues also identified the following list of mimics that can crop up:
- Mimics arising from gastrointestinal tract: Gastric pathology can extend into the left suprarenal space and mimic adrenal pathology. The most common mimics include gastrointestinal stromal tumors and gastric diverticula. Pathology elsewhere in the gastrointestinal tract can mimic adrenal pathology (e.g., a fluid-filled colon).
- Mimics arising from solid viscera: Pathology from the spleen, pancreas, liver, and kidneys can extend into the suprarenal space and mimic adrenal pathology. This includes splenic lobulation, splenunculi, upper pole renal pathology, pancreatic tail pathology, and exophytic hepatic lesions.
- Mimics arising from vessels: Dilated, tortuous, or aneurysmal vessels may extend into the suprarenal space and mimic adrenal pathology. The most common mimics include splenic varices and splenic artery pseudoaneurysms.
- Mimics arising from retroperitoneal tissues: Various retroperitoneal lesions can extend into the suprarenal space and mimic adrenal pathology, and normal anatomy in the retroperitoneum also can mimic adrenal pathology (e.g., a thickened diaphragmatic crus).