German researchers have found a way to monitor precisely how gadolinium-based contrast agents (GBCAs) enter the brain via the glymphatic pathway. This aspect has been overlooked and misinterpreted in the past, and the discovery may have important clinical implications, they emphasize.
In a study published online on 25 November by Investigative Radiology, Dr. Katerina Deike-Hofmann and colleagues from the German Cancer Research Center (DKFZ) at Heidelberg University Hospital and Essen University Hospital explain how gadolinium enters the cerebral spinal fluid (CSF). They show that GBCAs are excreted through specific entry points, such as the choroid plexus and the anterior chamber of the eye, into the CSF and subsequently enter the glymphatic system that, for instance, surrounds the perineural space.
"It is too early to conclude if this will be diagnostically meaningful, but we learned that there is a new imaging phase that we might have overlooked in recent years," study co-author Dr. Alexander Radbruch, JD, an associate professor of radiology and neuroradiology at Essen University Hospital and the DKFZ, told AuntMinnieEurope.com.
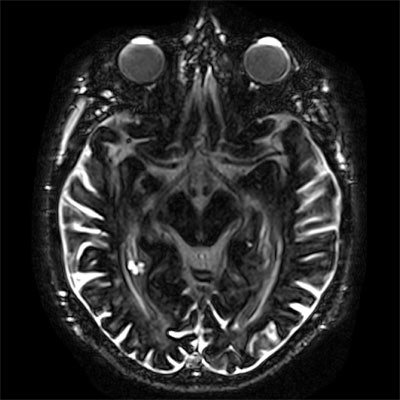
 Example of contrast enhancement three hours postinjection (3h p.i.) of a single dose of a gadolinium-based contrast agent on heavily T2-weighted fluid-attenuated inversion-recovery (FLAIR) imaging, compared with precontrast baseline in a 77-year-old man without any neurological disorder and normal renal function. a: Axial heavily T2-weighted FLAIR image on the level of the anterior eye segment at three hours postinjection demonstrates distinctive enhancement of the aqueous and vitreous chamber of the eyes, the distal optical nerve sheath, the choroid plexus, and the subarachnoid space compared with the precontrast baseline scan. b: Contrast enhancement of the prepontine cistern, fourth ventricle, subarachnoid space, cochlea, and Meckel's cave can be observed at three hours postinjection compared with the native baseline scan. Image courtesy of Dr. Alexander Radbruch, JD.
Example of contrast enhancement three hours postinjection (3h p.i.) of a single dose of a gadolinium-based contrast agent on heavily T2-weighted fluid-attenuated inversion-recovery (FLAIR) imaging, compared with precontrast baseline in a 77-year-old man without any neurological disorder and normal renal function. a: Axial heavily T2-weighted FLAIR image on the level of the anterior eye segment at three hours postinjection demonstrates distinctive enhancement of the aqueous and vitreous chamber of the eyes, the distal optical nerve sheath, the choroid plexus, and the subarachnoid space compared with the precontrast baseline scan. b: Contrast enhancement of the prepontine cistern, fourth ventricle, subarachnoid space, cochlea, and Meckel's cave can be observed at three hours postinjection compared with the native baseline scan. Image courtesy of Dr. Alexander Radbruch, JD.When they want to image the vessels, the Heidelberg researchers do it immediately after GBCA injection, but they wait some time for tumors to allow the GBCAs to distribute in the tumor tissue. For imaging of the glymphatic system, they just need to wait a little longer. Now the group thinks it is time to assess this approach systematically.
"Malfunctioning of the glymphatic system is supposed to be correlated with a plurality of neurological diseases, and now we might have a new imaging technique that can monitor it in vivo," Radbruch noted. "For imaging of the arteries, we do the MRI immediately after injection. For imaging of the tumor, we wait approximately five to 15 minutes to allow the gadolinium to enter the tumor. For imaging of the glymphatic system, we just need to wait some more time (three to 24 hours in our study). We never noticed this in the last 30 years because we did not examine patients in this time frame."
Gadolinium excreted in the glymphatic system might be regarded as a natural part of the excretion process, and the phenomenon should be differentiated from the issue of gadolinium deposition. This effect has been mixed up in the literature and is the major reason for some of the disagreements between European and U.S. researchers, according to Radbruch.
"GBCAs in the CSF might just take a little longer to be totally excreted, so we need to wait some time until we compare differences between the agents to not mix up permanent gadolinium deposition and gadolinium in the excretion process. This is why I also disagree with the definition of the ACR [American College of Radiology] consensus paper that defined gadolinium deposition as gadolinium in the brain after 24 hours," he added.
Heidelberg study details
Heavily T2-weighted fluid-attenuated inversion-recovery (FLAIR) MR images were obtained before and three hours and 24 hours after intravenous GBCA application in 33 neurologically healthy patients and seven patients with an impaired blood-brain barrier due to cerebral metastases. The Heidelberg team determined signal intensity in various cerebral fluid spaces and quantified white-matter hyperintensities by applying the Fazekas scoring system.
They used delayed heavily T2-weighted FLAIR to show GBCA entry into the central nervous system (CNS) via the choroid plexus and the ciliary body, with GBCA drainage along perineural sheaths of cranial nerves and along perivascular spaces of penetrating cortical arteries. In all patients and all sites, they found a significant signal intensity increase for the three hours and 24 hours time points compared with baseline.
Although the researchers found no significant difference in signal intensity between neurologically healthy patients and patients with an impaired blood-brain barrier, they identified a significant positive correlation between Fazekas scoring system and signal intensity increase in the perivascular spaces three hours postinjection.
Overall, the study reveals the potential of delayed gadolinium-enhanced MRI of the glymphatic system, and the findings have elucidated brain fluid dynamics and cerebral GBCA kinetics in vivo, the authors wrote. The findings have possible implications with respect to CNS drug delivery and imaging of impaired CSF microcirculation in a plurality of CNS diseases.