Can MRI function effectively without gadolinium? This question is more relevant than ever now, as the safety of gadolinium-based MRI contrast agents has become a hot topic and knowledge and awareness of gadolinium deposition have grown over recent years.
"I sometimes say radiologists have grown addicted to gadolinium," said Prof. Matthias van Osch, PhD, a professor in the radiology department at Leiden University. "We've introduced sequences at a time when gadolinium was supposed to be an absolutely safe contrast agent, and we've also got used to giving certain doses of contrast agent when, as a community, we've not tested whether these concentrations are really needed."
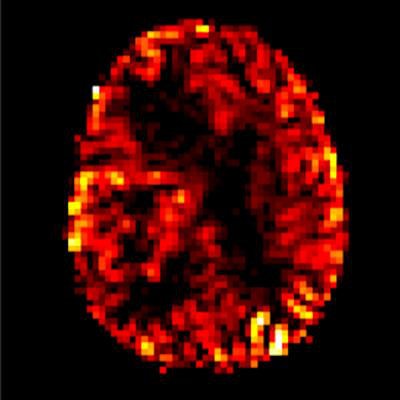
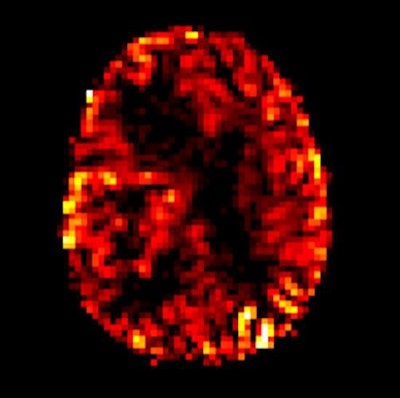 Arterial spin labeling perfusion imaging shows increasing blood flow in a malignant brain tumor in the right cerebral hemisphere. Image courtesy of Dr. Rolf Jäger.
Arterial spin labeling perfusion imaging shows increasing blood flow in a malignant brain tumor in the right cerebral hemisphere. Image courtesy of Dr. Rolf Jäger.Against this background, three experts discussed today whether gadolinium alternatives are ready now for clinical practice.
"Especially among patients, fear of contrast agents has increased considerably and there's quite a bit of worry in society about their effects," noted van Osch, who is co-moderator of the session.
He emphasized that gadolinium-based agents are not dangerous to use in healthy patients, as they aid diagnosis and any serious health risks have not yet been demonstrated. However, he also expressed concern that radiology is often slow to adopt alternatives to widely used techniques, and he hoped the session will help radiologists to find novel ways to reduce their gadolinium use.
Gadolinium-based contrast agents are a known trigger of nephrogenic systemic fibrosis, a rare disease that causes thickening of the skin and tissue of internal organs in patients with kidney disease. It is for this reason that these patients are in general not given gadolinium for MRI, van Osch explained.
His co-moderator, Prof. Dominique Sappey-Marinier, PhD, professor in biophysics, medical imaging, and neuroscience at the University of Lyon, France, noted that the main aim of the session was to explore all MRI techniques that allow the examination of the brain, heart, and other organs without using gadolinium-based contrast agents.
Dr. Rolf Jäger, professor of neuroradiology at the UCL Institute of Neurology, London, focused on gadolinium-free perfusion and angiography methods for imaging the brain. He covered arterial spin labeling (ASL), which magnetically labels protons in the patient's inflowing blood, thereby removing the need for injection of a contrast agent.
"With this method, we can look at blood flow in the large and medium-sized arteries of the brain and measure tissue perfusion at the capillary level. Emerging clinical applications are cerebrovascular diseases, brain tumors, as well as dementia and epilepsy," he said.
With 4D ASL angiography, radiologists can now obtain angiographic images of the cerebral vessels with high spatial and temporal resolution without using gadolinium. ASL perfusion imaging can measure cerebral blood flow and is likely to replace first-pass gadolinium bolus perfusion in many instances, but Jäger added that gadolinium is still needed for assessing a defective blood-brain barrier, which is important for imaging brain tumors.
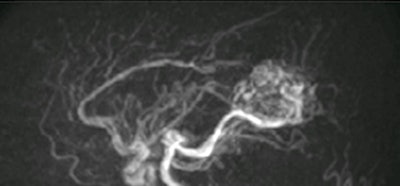 4D ASL angiography demonstrates the feeding arteries and nidus of a cerebral arteriovenous malformation. Image courtesy of Dr. Rolf Jäger.
4D ASL angiography demonstrates the feeding arteries and nidus of a cerebral arteriovenous malformation. Image courtesy of Dr. Rolf Jäger.Dr. Bettina Baeßler, an associate professor of radiology at the University Medical Center Mannheim, Germany, presented alternatives to gadolinium-based contrast agents for cardiac and prostate applications. Replacing gadolinium in these areas can be harder than in the brain.
"The heart moves, so acquisition of images is technically much more challenging than in the brain," she said. "For prostate imaging, movement is not as important as the heart, but also there, the bowel and the rectal wall are more or less moving."
Movement of the rectal wall can sometimes lead to distortions of diffusion-weighted MR images, and the presence of air within the rectum can also degrade image quality, which makes it harder to replace gadolinium-based contrast agents, she explained.
Multiparametric MRI in combination with artificial intelligence (AI) is a very promising alternative to gadolinium-based agents and Baeßler noted that some multiparametric MRI methods are already widely used in clinical practice.
A combination of mapping and radiomics has huge potential as an alternative to gadolinium, she continued. Mapping techniques (i.e., MR relaxometry) yielding absolute quantitative T1, T2, or T2* relaxation times are already a good alternative to using gadolinium-based contrast agents for the assessment of the pattern of iron deposition in the myocardium or in Fabry disease, for instance. However, they need to overcome several hurdles before they can replace gadolinium when assessing other conditions, such as myocarditis and myocardial fibrosis.
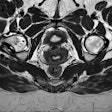
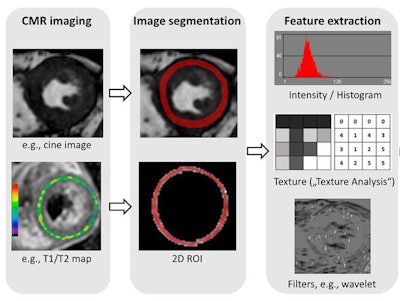 Radiomics in cardiac MR (CMR). Radiomic feature extraction can be performed on all types of MR images, such as cine images or T1/T2 maps. Image courtesy of Dr. Bettina Baeßler.
Radiomics in cardiac MR (CMR). Radiomic feature extraction can be performed on all types of MR images, such as cine images or T1/T2 maps. Image courtesy of Dr. Bettina Baeßler.The major hurdle, Baeßler argued, is standardization of quantitative imaging techniques, including mapping and diffusion-weighted imaging (DWI). Values of myocardial T1/T2 or apparent diffusion coefficient (ADC) can vary between MRI sequences, instrument manufacturers, and field strengths. There is also a large natural variation in T1 and T2 relaxation times in the hearts of healthy individuals, which makes it hard to set a cut-off for distinguishing healthy and diseased tissue.
To get around this problem, Baeßler suggested using radiomics in conjunction with quantitative MRI techniques. Radiomics can identify tissue inhomogeneity and other features in MRI, but it also faces issues with standardization. Radiomics-derived, or even AI-derived, features currently depend on factors like MR signal intensity.
"The work on standardization of radiomic feature extraction is the most important point for radiomics right now, before it will become a real alternative to gadolinium in clinical practice," she concluded.
Originally published in ECR Today on 3 March 2019.
Copyright © 2019 European Society of Radiology