Noninvasive medical imaging is an essential part of the diagnostic workup and follow-up of patients. Standard imaging modalities include transmission tomography (e.g., CT or conebeam CT [CBCT]), nuclear medicine methods (e.g., PET), or MRI. Lately, hybrid imaging technologies have been introduced that combine both PET and CT or MRI. Noninvasive imaging methods are used, for example, for staging oncology patients, whereby helpful information on the tumor stage, extent, and even grade can be acquired through the use of different imaging methods.
Take-away messages
- Involuntary patient motion is observed in multiple noninvasive imaging exams that precede personalized treatment planning and treatment effect monitoring.
- To date, various prospective and retrospective patient motion management strategies exist that aim primarily at addressing rigid motion (e.g., head movement) or image distortions from cardiac and respiratory motion.
- Other, less frequently observed motion effects from, for example, swallowing or torso movement, are more complex to address with standard motion management strategies today.
- Current patient motion management strategies yield improved image quality by reducing image blurring and increased quantitative accuracy, however, few larger scale clinical studies are available to assess the impact on diagnostic accuracy.
- Especially for PET, motion correction is important to be able to use all acquired data to achieve sufficient signal-to-noise ratio while minimizing motion artifacts.
Such imaging methods are employed also for assessing neurodegenerative disease and cardiovascular diseases. Irrespective of the clinical indication, imaging procedures take time from a second, or so, up to an hour. In most cases, image acquisition extends over several involuntary cardiac or respiratory cycles of the patient. Moreover, these cycles can be aperiodic given the cause of the disease or the general state patients are in (e.g., mental stress, overall well-being, etc.).
Depending on the magnitude and frequency, involuntary patient motion may lead to reduced image quality through blurring of tissue boundaries as well as to quantitative effects and biases, if quantitative measures, such as average tissue density (e.g., Hounsfield units), standardized uptake values (SUV), coronary vessel narrowing, etc. are employed. Both visual and quantitative detriments can impact patient diagnosis and treatment. It is, therefore, of great interest to detect image artifacts, and, if possible, to correct for them.
Patient motion management is, thus, essential in state-of-the-art imaging. At the recent ECR in Vienna, imaging experts reviewed motion management strategies for some of the most challenging cases, such as cardiac motion in CT, respiratory motion in CBCT as part of patient setup strategies in external radion therapy, and, finally, in cardiac imaging using combined PET/MRI.
Managing motion in CT: Conventional approaches and motion compensating techniques
CT is a very fast imaging modality -- with scan times in the order of seconds -- with a very high temporal resolution (about 140 msec for single-source CT and about 70 msec for dual-source CT). Nonetheless, patient motion may lead to motion artifacts, which may result in simple motion blurring, or so-called curved or trochoid artifacts, in case details move at very high speed, such as in the heart (figure 1A).
These artifacts are prominent in cases when patient preparation was not done correctly, when the heart rate is highly variable, or when the gating window does not fall into the best phase. In these cases, new mathematical methods are available that estimate the motion of each voxel and account for that motion (velocity and direction) during image reconstruction. These motion-compensation (MoCo) algorithms have the potential to increase the temporal resolution by about one order of magnitude (figure 1B).
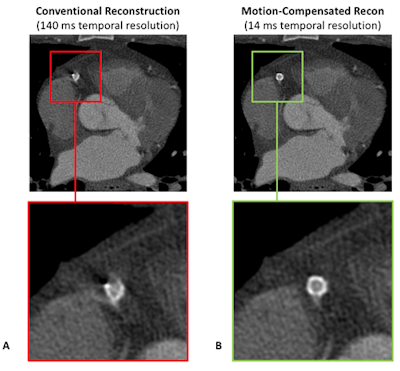 Figure 1: Although the temporal resolution is quite high, the coronary stent is not well visualized in the conventional reconstruction (A). The motion-compensated reconstruction, which mathematically models the motion of each voxel and accounts for that motion during image reconstruction, achieves a 10-times higher temporal resolution, and, thus, correctly depicts the implant (B).
Figure 1: Although the temporal resolution is quite high, the coronary stent is not well visualized in the conventional reconstruction (A). The motion-compensated reconstruction, which mathematically models the motion of each voxel and accounts for that motion during image reconstruction, achieves a 10-times higher temporal resolution, and, thus, correctly depicts the implant (B).In contrast, respiratory motion is not a significant problem in CT, due to the short scan times; here, breath-holds are very short or not required at all. However, when respiratory motion shall be resolved, such as for radiation therapy planning, then dedicated slow CT scans with a low pitch value are performed. In these cases, missing data artifacts are frequently observed due to irregular breathing, which could, in principle, be compensated by dedicated algorithms. Such algorithms, however, are not provided by the vendors as of today.
Managing motion in CBCT: Conventional approaches and motion compensating techniques
CBCT is highly affected by patient motion because of the relatively slow rotation speed compared with conventional fanbeam CT. The management of the motion depends on the clinical use case, whereby the suppression of image artifacts or the resolution of patient motion is preferred. In particular, in the field of image-guided radiation therapy (IGRT), knowledge of the absolute geometric position of target structures is essential for optimum treatment beam delivery. Current solutions focus mainly on patient immobilization or breath-hold. Nonetheless, in patients with lung cancer, motion effects cannot be avoided, and proper consideration must be given to advanced motion management techniques in light of patient comfort and clinical workflow aspects.
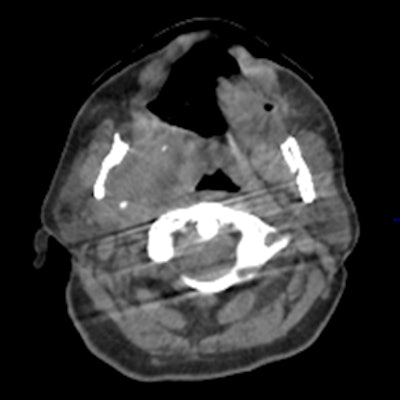
To date, all state-of-the-art motion management approaches in CBCT image reconstruction have a number of limitations; for example, immobilization devices (e.g., head frames) do not exclude patient motion entirely, and despite their rigidity and patient discomfort still permit for residual involuntary patient motions, such as swallowing or residual flexibility (figure 2).
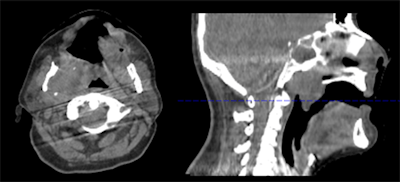 Figure 2: Motion artifact in head scan with head frame immobilization originating from residual motion of the spine.
Figure 2: Motion artifact in head scan with head frame immobilization originating from residual motion of the spine.Typically, such nonperiodic motion affects only a subset of the x-ray projections but impairs overall image quality. For abdominal therapy planning using CBCT, prospective gating or acquiring a single or a sequence of breath has been demonstrated to improve image quality. In temporally resolved CBCT, clinically available algorithms are usually based on retrospective phase gating. This implies that each 3D phase image is reconstructed from a subset of the x-ray projections, and, therefore, most likely suffers from sparse data artifacts. However, MoCo reconstruction (figure 3) provides the unique advantage to use all projections for the image reconstruction of each phase.
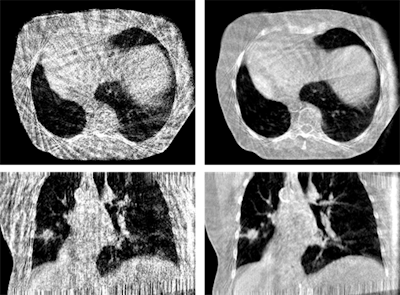 Figure 3: Phase-correlated (left) and motion-compensated (right) reconstruction of a thorax scan. The MoCo reconstruction is constrained by cyclic motion and uses an artifact model and phase to amplitude resampling to improve image quality.
Figure 3: Phase-correlated (left) and motion-compensated (right) reconstruction of a thorax scan. The MoCo reconstruction is constrained by cyclic motion and uses an artifact model and phase to amplitude resampling to improve image quality.One of the main challenges in MoCo reconstruction is the coregistration step between the phase reconstructions. Such coregistration must be insensitive to the quality of the phase-correlated image reconstructions, and it must be able to handle sliding organ motion and incompressibility of bones. Moreover, all time-resolved reconstruction approaches have in common that they rely on regular breathing amplitudes. Recently published work addresses the irregularity in breathing patterns by performing phase-to-amplitude resampling.
Motion compensation in MR and PET imaging
Dual-modality PET/MRI combines the high sensitivity of PET with the breadth of image contrasts from MRI. In particular, for oncological applications, anatomical MR images with high soft-tissue contrast provide important diagnostic information complementing that of the metabolic PET information. In contrast to PET/CT, fully integrated PET/MRI systems provide a platform for the simultaneous acquisition of both image information, which, in addition to advanced image fusion, offers new possibilities for motion management.
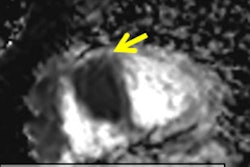
Several in vivo studies have demonstrated the displacement of organs due to breathing or heartbeat can be accurately determined in MR images, and this information can then be used to improve the quality and quantitative accuracy of complementary PET data. Subsequently, these corrections lead to an increase of the measured PET uptake signal between 20% to 40%. PET/MRI also allows for the combination of PET and MRI data to achieve more accurate estimation of motion displacement (figure 4). Here, respiratory and cardiac motion correction leads to an improved visualization of F-18 FDG PET uptake in the heart and increases the measured PET uptake values by more than 25%. So far, the feasibility of motion correction in PET/MRI has been demonstrated, while studies with higher patient numbers are required now to assess the potential for clinical improvement of the diagnostic accuracy.
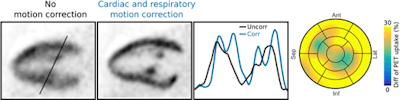 Figure 4: Long-axis slices showing F-18 FDG PET uptake in the myocardium and papillary muscles without motion correction and with cardiac and respiratory motion correction. Improved depiction of the uptake structures is also visible in the line plot. Motion correction improved the measured uptake by more than 25% on average.
Figure 4: Long-axis slices showing F-18 FDG PET uptake in the myocardium and papillary muscles without motion correction and with cardiac and respiratory motion correction. Improved depiction of the uptake structures is also visible in the line plot. Motion correction improved the measured uptake by more than 25% on average.Thomas Beyer, PhD, is with the Medical University Vienna Center for Medical Physics and Biomedical Engineering, Quantitative Imaging and Medical Physics Team. Dr. Pascal Paysan is with Image Enhancement and Reconstruction, Varian Medical Systems Imaging Laboratory in Baden-Dättwil, Switzerland. Christoph Kolbitsch, PhD, is head of Research Group Quantitative MRI Physikalisch-Technische Bundesanstalt in Berlin. Dr. Marc Kachelriess, PhD, is with the Division of X-Ray Imaging and CT at the German Cancer Research Center (DKFZ) Foundation under Public Law in Heidelberg, Germany.
The comments and observations expressed herein do not necessarily reflect the opinions of AuntMinnieEurope.com, nor should they be construed as an endorsement or admonishment of any particular vendor, analyst, industry consultant, or consulting group.