Two widely used linear gadolinium MRI contrast agents will be pulled from the U.K. market by 1 February, while the use of other linear agents will be restricted to limited indications, according to an announcement by the U.K.'s Medicines and Healthcare products Regulatory Agency (MHRA).
In a safety update issued on 14 December, the MHRA announced the marketing licenses for the linear gadolinium agents gadodiamide (Omniscan, GE Healthcare) and intravenous gadopentetic acid (Magnevist, Bayer HealthCare Pharmaceuticals) would be suspended in February and the products would be recalled. The clinical indications for two other linear gadolinium agents, MultiHance (Bracco) and Primovist (Bayer) will be changed to limit their use to delayed-phase MR imaging of the liver.
The move essentially implements action taken by the European Medicines Agency (EMA), which earlier this year ruled that Omniscan and Magnevist, as well as a third agent, Optimark by Guerbet, should be removed from the European market. The European Commission gave European Union member states a year to implement the decision in their individual markets, but the MHRA has apparently decided on a more aggressive timeline for the U.K. (The MHRA noted that Guerbet has already withdrawn Optimark from the U.K.)
In announcing the decision, the MHRA said it expected healthcare providers to have switched over to alternative gadolinium MRI contrast products by the February deadline. The agency noted the macrocyclic agents Prohance (Bracco), Gadovist (Bayer), and Dotarem (Guerbet) will remain on the market; gadopentetic acid will remain authorized for intra-articular use, the safety update stated.
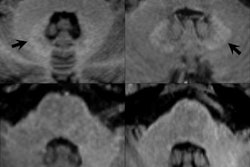
The MHRA explained that use of linear gadolinium agents has "decreased markedly" in the U.K. since 2006 in favor of macrocyclic products following warnings about the link between the agents and nephrogenic systemic fibrosis (NSF) in some patients. But new research confirming the presence of low levels of gadolinium deposition in the brain in patients who received MRI agents has reopened questions about the safety of the agents. Macrocyclic MRI agents are believed to have much lower potential to cause gadolinium retention than linear agents because they are more stable.
"In view of the evidence of retention of gadolinium in brain and other tissues following exposure to these agents, the risks of gadodiamide and intravenous gadopentetic acid are considered to outweigh their benefits," the MHR update reads.
The safety update can be viewed on the agency's website.