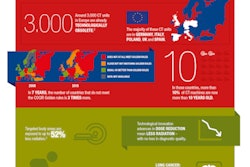
The European Coordination Committee of the Radiological, Electromedical, and Healthcare IT Industry (COCIR) has given its blessing to the new regulatory framework for medical devices, the Medical Device Regulation (MDR), which was approved in a vote on 5 April during a plenary session of the European Parliament.
The MDR is the result of four years of debate on updating the continent's legislative framework for medical devices to keep pace with new technological changes. It is designed to promote and advance safety improvements of medical devices through features such as a unique identification number for medical devices and a centralized database.
COCIR stated the MDR will support a transparent regulatory framework and enhance the ability to trace equipment through unique identification numbers and a readily accessible central database.