The development of more efficient MRI systems and faster imaging techniques means that a streamlined MR protocol with an image acquisition time of only six minutes can become the gold standard for initial assessment of acute stroke, according to new international research.
"With the new generation of high-performance scanners and faster imaging sequences, MRI can become the standard for the initial evaluation of acute stroke," noted Dr. Asik Ali Mohamed Ali, assistant professor of diagnostic imaging at Saveetha Medical College Hospital in Chennai, India, and a cardiothoracic imaging researcher at the University of British Columbia in Vancouver.
Compared with CT, MRI is much more accurate when it comes to deciding on patient management, added Ali and colleagues, whose e-poster received a certificate of merit at ECR 2016 in Vienna.
CT has become the major workhorse in the initial assessment of acute stroke because of the faster acquisition time due to the advent of multidetector technology, the modality's widespread availability, and the ease of interpretation in the emergency setting, but now MRI is showing great promise because it is very sensitive and more specific in the delineation of infarcted core volume when compared with CT, they explained.
A comprehensive MR protocol includes parenchymal imaging, MR angiography, and MR perfusion with the acquisition time of 20 minutes, but this can be reduced drastically (see flowchart).
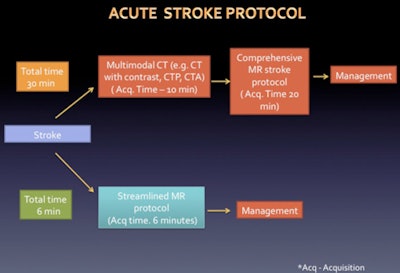 Overview of stroke protocol. Source: Radiology & Imaging, Saveetha Medical College Hospital, Chennai, India, presented at ECR 2016.
Overview of stroke protocol. Source: Radiology & Imaging, Saveetha Medical College Hospital, Chennai, India, presented at ECR 2016.Both CT and conventional MR sequences can help to establish and diagnose acute stroke as early as possible, and information about the intracranial vasculature and brain perfusion also can be obtained, yet these sequences do not aid in differentiating ischemia from infarcted tissue, the authors stated.
"Reperfusion of this ischemic tissue can prevent serious disability and significantly improve a patient's quality of life," they continued. "Advanced sequences such as diffusion-weighted and perfusion imaging help in detecting hyperacute infarct as well as ischemic (penumbra) tissue within the short therapeutic window time. With the advancement of aggressive and promising new therapies on hand, early recognition of ischemia places diagnostic neuroimaging at the forefront of stroke management."
Time lost is brain lost
Stroke is not an event, but a multistep process, and it's essential to remember that time lost is brain lost, Ali et al pointed out. A cascade of events occurs when there is a decrease in cerebral perfusion, and the radiologist must collaborate with the referring physician and neurologist and tailor the imaging protocol. Customized protocols will help in starting the reperfusion therapy at the very initial stage with considerable amount of window time.
Referring to the "4 Ps," they explained that imaging has four main goals: parenchyma (assess early signs of acute stroke, rule out hemorrhage; pipes (assess extracranial circulation [carotid and vertebral arteries of the neck] and intracranial circulation for evidence of intravascular thrombus); perfusion (assess cerebral blood volume, cerebral blood flow, and mean transit time); and penumbra (assess tissue at risk of dying if ischemia continues without recanalization of intravascular thrombus).
A patient whose clinical condition is critical is easier to monitor in a CT scanner in comparison with MRI, for which special equipment is required, and a hemorrhage is easier to detect with CT, the authors wrote. However, CT has several important limitations: it does not reliably detect infarcts of less than four hours duration, the extent of the infarct is often difficult to characterize, and acute lacunar infarcts often go undetected and are impossible to distinguish from chronic lacunar infarcts. Furthermore, unenhanced CT provide only limited information about intracranial vessels and status of the brain that surrounds already infarcted tissue, and the introduction of low-dose algorithms to limit radiation exposure to boost patient safety increases the noise, which makes subtle infarct changes more difficult to detect.
An investigation using comprehensive MR stroke protocols takes around 20 minutes and involves parenchymal imaging (infarcted core and hemorrhage), MR angiography (arterial occlusion and intravascular thrombus), and perfusion imaging to detect hypoperfused tissue and penumbra. Therefore, the sequences in these protocols include diffusion-weighted imaging, fluid attenuated inversion recovery (FLAIR) to age the infarct, gradient recalled echo (GRE) to detect parenchymal hemorrhage, time-of-flight MR angiography, and dynamic susceptibility contrast (DSC).
In both contrast-enhanced MR angiography (CEMRA) and DSC perfusion imaging, administration of a single dose of contrast is used, they continued. Twenty ml of gadolinium is diluted with normal saline to a total 50 ml volume, and using a timing bolus, a total of 3 ml of contrast solution (1.2 ml of gadolinium) is injected at 1.5 ml/s to determine the transit time from the arm vein to the cervical carotid arteries. Then, a total of 22 ml contrast solution (8.8 ml of gadolinium) is injected at the same flow rate as the timing injection for the CEMRA acquisition. The remaining 25 ml of contrast solution (10 ml of gadolinium) is injected at 5 ml/s for the MR perfusion scan.
Postprocessing imaging analysis is performed on the workstation with dedicated neuro software to obtain the parametric perfusion maps and to analyze diffusion-perfusion mismatch, the authors concluded.
To view the authors' clinical cases presented at ECR 2016, click here.
Editor's note: The diffusion-weighted image on the home page of AuntMinnieEurope.com is of a 55-year-old man presenting with right-sided hemiparesis and aphasia. MRI shows a relatively small final infarction volume. Image courtesy of Dr. Wolfgang Kunz, Institute of Clinical Radiology at Ludwig Maximilian University of Munich.