With steeper dose gradients than x-rays, protons have the potential to target tumors more precisely and better spare healthy tissue. However, to exploit proton therapy's full potential, imaging that enables accurate treatment guidance and dose calculation is particularly important. In a departure from x-ray imaging techniques currently used, a select few research groups are using simulations to explore the potential of a hybrid MR-guided proton therapy system.
 First author Joris Hartman, from the University Medical Center Utrecht.
First author Joris Hartman, from the University Medical Center Utrecht.In the latest study, researchers in the Netherlands and U.S. have successfully generated intensity-modulated proton therapy (IMPT) plans in the presence of a diagnostic-strength magnetic field. Achieved with inverse optimization and Monte Carlo calculations, dose coverage of the target was similar to that generated in the absence of a magnetic field, in findings that justify future research into a clinical MR-proton therapy hybrid system.
"Our study showed that the dose distributions are equivalent inside the target and that MR-guided proton therapy is dosimetrically feasible," said first author Joris Hartman of the University Medical Center Utrecht (Physics in Medicine and Biology, 7 August 2015, Vol. 60:15, pp. 5955-5969). Led by senior authors Jan Lagendijk and Bas Raaymakers, UMC Utrecht researchers carried out the work in collaboration with the University of Texas MD Anderson Cancer Center. Both centers are part of a consortium developing an MRI-linac set for a commercial launch in 2017.
According to the Lorentz force law, a magnetic field will deflect a proton pencil beam along a curved path and the researchers sought to quantify the exact impact of a 1.5-tesla field on an IMPT treatment plan for the first time. In their simulation, the patient was positioned parallel to the field, as if in the bore of an MRI scanner. Beamlets were fired at right angles to the field and the patient axis, simulating a worst-case deflection geometry. A treatment system was not simulated, though researchers at the two centers have since begun to develop a model as part of their ongoing research.
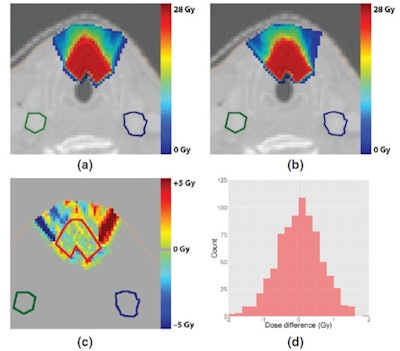 The head-and-neck tumor treatment plan generated without a magnetic field (top left) was compared with the plan generated with a 1.5-tesla field (top right). A plot and histogram (bottom row) of the dose difference between the two shows equivalent target coverage. All images courtesy of Joris Hartman.
The head-and-neck tumor treatment plan generated without a magnetic field (top left) was compared with the plan generated with a 1.5-tesla field (top right). A plot and histogram (bottom row) of the dose difference between the two shows equivalent target coverage. All images courtesy of Joris Hartman.Three previously treated cases were planned with a fixed number of beams at fixed angles using CT scans: a head-and-neck tumor whose center was 2 cm deep, a head-and-neck tumor whose center was 4 cm deep, and a simulated liver tumor 9 cm deep. In each case, the plan optimizer adapted for proton deflection by selecting different beamlets, with more marked differences for the deeper-seated tumors that experience greater beam deflections.
Comparison of the plans with and without the magnetic field revealed mean dose differences in the gross tumor volume (GTV) of 0.018 Gy (standard deviation 0.65 Gy) and -0.17 Gy (standard deviation 1.11 Gy) in the head-and-neck cases. In the liver case, the mean dose difference was -0.34 Gy (standard deviation 0.62 Gy). As beam angles were chosen to avoid organs-at-risk (OAR), beamlet deflection had no significant effect on the small doses they received. Low doses received by the rest of the body were simply shifted to other locations by the deflections.
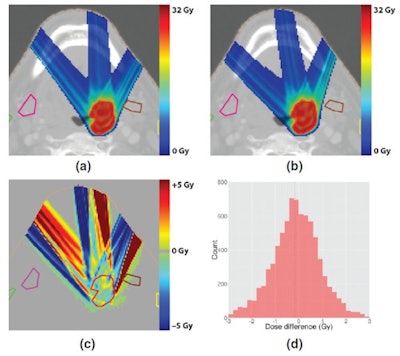 Comparison of treatment plans for a deep head-and-neck tumor, generated without a magnetic field (top left) and with a 1.5-tesla field (top right). A plot and histogram (bottom row) of the dose difference between the two shows equivalent target coverage, but bigger differences than for the shallow tumor.
Comparison of treatment plans for a deep head-and-neck tumor, generated without a magnetic field (top left) and with a 1.5-tesla field (top right). A plot and histogram (bottom row) of the dose difference between the two shows equivalent target coverage, but bigger differences than for the shallow tumor.While the results are a step in the right direction, Hartman estimates that it could be 10 to 20 years before MR-guided proton therapy is available to patients. Technical challenges associated with combining the two systems will need to be overcome and collaboration with industry will be "essential."
Prior to that, further simulation studies are also needed to assess which treatment sites can benefit from the superior soft-tissue contrast and guidance MR imaging affords over 2D and 3D x-ray imaging techniques. More fundamentally, MR imaging's ability to accurately determine stopping powers and proton range, both in treatment planning and in real-time when the patient is on the treatment couch, will also need to be assessed.
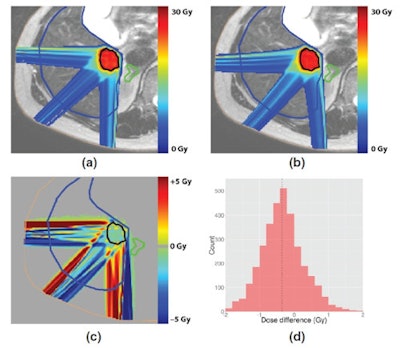 Comparison of a liver tumor treatment plan generated without a magnetic field (top left) and a plan generated with a 1.5-tesla field (top right). A plot and histogram (bottom row) of the dose difference between the two shows equivalent target coverage, as for the other two cases.
Comparison of a liver tumor treatment plan generated without a magnetic field (top left) and a plan generated with a 1.5-tesla field (top right). A plot and histogram (bottom row) of the dose difference between the two shows equivalent target coverage, as for the other two cases."We want to study what the potential benefits are of MR-guidance in clinical proton therapy," Hartman said. "We have to answer those questions first before we can think of developing a physical system." The collaboration plan is to investigate these issues with the modelled system that they are developing.
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.