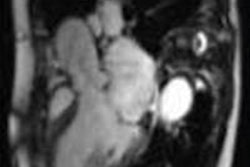
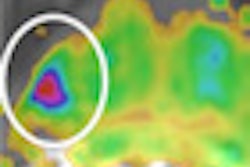
In the appropriate clinical setting, cerebral fat embolism (CFE) can be differentiated from diffuse axonal injury because CFE has a higher incidence of increased T2-FLAIR and diffusion-weighted imaging (DWI) lesions, smaller hemorrhages, and does not exhibit linear subcortical hemorrhages that are seen with diffuse axonal injury, according to a global team of MRI researchers.
CFE in the trauma setting can mimic the conventional MRI findings seen with diffuse axonal injury, and both can result in areas of white matter diffusion restriction, T2 white-matter signal abnormality, and extensive cerebral microhemorrhages, noted lead author Dr. Manal Ahmed El Refaei, a radiologist at Al Ahrar General Hospital, Sharkia, Ministry of Health, Egypt, in an e-poster presented at last week's International Congress of Radiology (ICR) in Dubai, United Arab Emirates. Her four co-authors of the study were from the University of Washington (Kirkland and Seattle) and Johns Hopkins University in Baltimore.
CFE is a serious complication after fracture of long bones. The mortality of CFE may be high, but it is a potentially reversible disease, given recent progress in treatments, the author explained. Though MRI findings are definite in a given clinical setting, they alone are not specific and can be observed in diffuse axonal injury.
Cerebral fat embolism syndrome mimics diffuse axonal injury on MRI with vasogenic edema, cytotoxic edema, and microhemorrhages, making specific diagnosis a challenge. However, susceptibility-weighted imaging, an MRI technique sensitive to magnetic susceptibility effects, is beneficial in detecting intravenous deoxygenated blood as well as extravascular blood products.
"It is crucial to set down definite imaging criteria for trauma patients to enable appropriate differential diagnosis," they stated.
They conducted a retrospective study of 200 patients referred from the emergency room to the radiology department in Harborview Medical Center, Seattle, where nine patients with CFE and nine patients with diffuse axonal injury were selected. All patients were imaged on a 1.5- or 3-tesla MRI system, including conventional sequences and susceptibility-weighted imaging.
The inclusion criteria were as follows:
- CFE: long bone fractures, Glasgow Coma Scale (GCS) of 15 or 11T, no loss of consciousness at time of injury, and suffered a latent decline of GCS to ≤ 6T
- Hemorrhagic diffuse axonal injury: no long bone fractures, GCS at presentation of ≤ 6T, without a latent decline in GCS
- Normal presenting head CT, while MRI revealed presence of multiple microhemorrhages in a post-traumatic patient
The T2, FLAIR, DWI, and susceptibility-weighted imaging sequences were evaluated for lesions at the juxtacortical and subcortical areas, as well as the deep and periventricular white matter of the frontal, parietal, occipital, and temporal lobes, as well as genu, body, and splenium of the corpus callosum, midbrain, pons, medulla, and cerebellum. The lesions' signal pattern, number of lesions, and the hemorrhagic lesion's location, size, and shape were evaluated.
At each station, lesions were counted and categorized based on whether there was 0, 1-2, 3-5, or more than five lesions. For the whole brain, the lesions were also counted and categorized based on whether there was 0, 1-2, 3-5, 6-20, 21-100, or more than 100 lesions.
In addition, the size and configuration of the microhemorrhages were documented, based on the following criteria: punctuate < 3, small 4-10, medium 11-20, lobar > 100 lesions. The Mann-Whitney test was performed for the total number of brain lesions for each sequence.
There were significantly more hemorrhagic lesions in CFE relative to diffuse axonal injury at the following white matter stations: parietal periventricular (p = 0.0011), deep white matter (p = 0.0061), cerebellum (p = 0.001), and brainstem (p = 0.012). No significant difference in the corpus callosum was reported.
Beginner's guide to susceptibility-weighted imaging
Another e-poster at ICR 2014 focused on the basics of susceptibility-weighted imaging.
It is mainly used for visualization of hemorrhage, quantification of iron content in the basal ganglia, and visualization of brain calcifications, according to Dr. Taha M. Mehemed, research fellow and PhD candidate at the department of diagnostic imaging and nuclear medicine at Kyoto University in Japan, and colleagues. Originally developed as a brain venography technique, relying on susceptibility effects of venous deoxyhemoglobin (blood-oxygen level dependent [BOLD] venography), it consists of the original magnitude image multiplied by a high-pass filtered phase mask image.
"Both diamagnetic and paramagnetic substances appear as low signal on susceptibility-weighted imaging," they noted. "High-pass filtered phase mask image contrast can differentiate between diamagnetic and paramagnetic substance through their opposing dipole appearance."
The main points to bear in mind with susceptibility-weighted imaging are:
- Orientation of structures within main magnetic field effect the appearance on the high-pass filtered phase mask image and might alter the final appearance on susceptibility-weighted imaging
- Geometry and shape of structures/lesions affect high-pass filtered phase mask images
- Venous blood oxygen level affects appearance on susceptibility-weighted imaging; the more oxygenated the blood, the more diamagnetic and the less the contrast with background
- Low on susceptibility-weighted imaging doesn't always mean hemorrhage