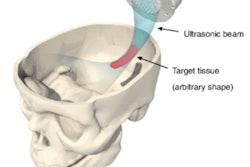
The blood-brain barrier (BBB) prevents most drugs from entering the brain and, thus, is a major obstacle in the use of chemotherapy to treat brain tumors. Applying focused ultrasound pulses, combined with microbubble-based ultrasound contrast agents, can temporarily open up the barrier and enable drug penetration.
While extracranial ultrasound can achieve such an opening, ultrasound energy is highly attenuated by the skull bone. The skull also defocuses and laterally shifts the ultrasound beam, resulting in an uncertain focal point. To overcome this hurdle, researchers at French start-up company CarThéra are developing an implantable ultrasound device, called SonoCloud, which enables transient and repeated opening of the blood-brain barrier.
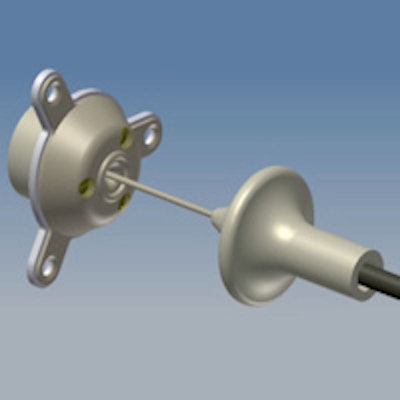
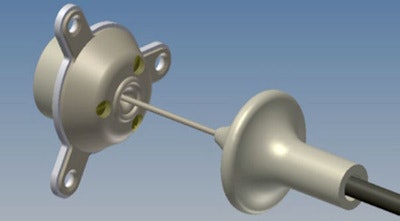 The implantable SonoCloud device is powered by an external generator via a transdermal needle connection.
The implantable SonoCloud device is powered by an external generator via a transdermal needle connection.At the recent American Association of Physicists in Medicine (AAPM) annual meeting in Indianapolis, Indiana, Cyril Lafon, PhD, the research director from the French Institute of Health and Medical Research's (INSERM) Laboratory of Therapeutic Applications of Ultrasound (LabTAU), updated delegates on progress to date. Lafon, one of CarThéra's co-founders, explained that brain tumors are commonly treated using surgical excision followed by chemotherapy. The SonoCloud device is designed to be implanted into the skull bone (via a burr hole) during the initial surgery. It can then be employed at all subsequent chemotherapy sessions to open the blood-brain barrier and optimize intracerebral drug concentration.
Initial testing
SonoCloud is a 10-mm-diameter, 1-MHz ultrasound transducer. It has no internal energy source, making it compatible for use in MRI systems, and is instead powered by an external generator via a transdermal needle connection that's plugged in only during treatment sessions. The technique was originally proposed by Dr. Alexandre Carpentier, a neurosurgeon at l'Hôpital de la Pitié-Salpêtrière in Paris.
Lafon first described some of the preclinical research performed to assess the short-term safety of the device. In one recently published study, Lafon and colleagues used the transducer, along with intravenous injection of SonoVue contrast agent, to perform blood-brain barrier opening in 16 rabbits (Journal of Neurosurgery, 21 June 2013). Opening was confirmed by detecting extravasation of blue dye into the brain tissues.
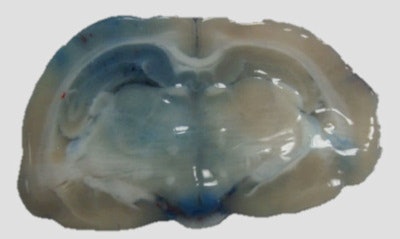 Extravasation of blue dye into brain tissues is observed in the left side of the brain that was sonicated with ultrasound using the SonoCloud device.
Extravasation of blue dye into brain tissues is observed in the left side of the brain that was sonicated with ultrasound using the SonoCloud device.A toxicity assessment performed seven days after the opening revealed limited damage to healthy tissue, with minor adverse effects and moderate edema limited to the extent of the sonication field. A similar study on dogs revealed no adverse effects (using either MRI or histology) and no signs of behavior modification. MR images recorded at day seven revealed a normal blood-brain barrier.
The researchers also performed studies to evaluate uptake in the rabbit brain of two chemotherapy drugs: temozolomide -- a small drug used to treat glioma that can traverse the BBB, and an alternative drug, irinotecan. Five minutes after chemotherapy administration, they used the ultrasound device to open the BBB. A 20% increase in uptake of temozolomide was seen in the "ultrasonicated" brain hemisphere, compared with the contralateral control hemisphere. The results for irinotecan were more pronounced, with an increased uptake of 206% following ultrasound exposure.
Long-term safety
Lafon went on to describe the current work being performed to evaluate the long-term safety and potential toxicity of SonoCloud. For this study, the researchers implanted the ultrasound device in the skulls of three primates, using a typical neurosurgeon's burr hole. Blood-brain barrier opening was achieved by applying 1-MHz ultrasound pulses with 25-msec pulse length and a pressure of 0.6-0.8 MPa for 120 sec, along with administration of SonoVue contrast agent.
The team performed BBB openings in the primary motor cortex every two weeks over a three-month period (a total of seven openings). Contrast-enhanced MRI revealed BBB opening immediately after each sonication. Seven days later, PET scans with the radiotracer DPA-714 revealed no sign of inflammation.
The animals were followed up for four months, and all exhibited normal behavior during this period. Electrophysiology follow-up revealed no signs of epilepsy, cognitive decline, or medicinal encephalopathy. FDG-PET scans showed no significant changes in cerebral glucose metabolism, while histology showed no signs of hemorrhagic processes or ischemia, and no change in the vessel wall integrity.
Lafon concluded that SonoCloud is a promising and safe ultrasound device for repeated blood-brain barrier opening in patients undergoing chemotherapy following surgical excision of brain tumors.
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.