Dynamic perfusion CT is a young technique with a few limitations, but it can usually distinguish normal from ischemic myocardium -- and reliably separate myocardial infarction from ischemia, concluded a leading German expert at the 2013 International Symposium on Multidetector-Row CT.
In a small study of patients who underwent stress dynamic dual-source CT angiography along with rest and stress cardiac MR (CMR) for confirmation, CT showed good diagnostic accuracy versus MRI in terms of its ability to measure blood flow and volume quantitatively at fairly low radiation doses, said Dr. Konstantin Nikolaou, a professor and the vice chair of clinical radiology at University Hospitals Munich.
The first dynamic perfusion CT studies have been published only in the last couple of years, and now efforts are focused on determining what clinical questions it can answer.
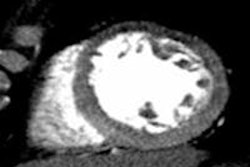
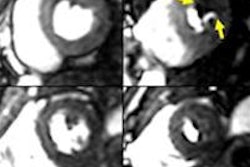
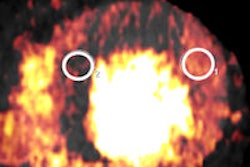
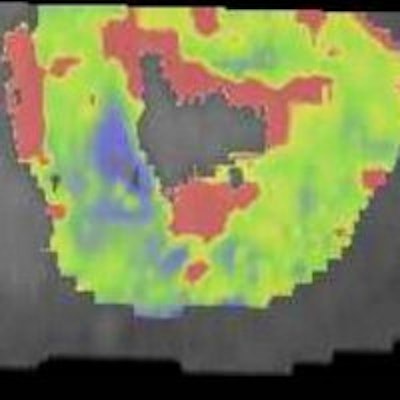
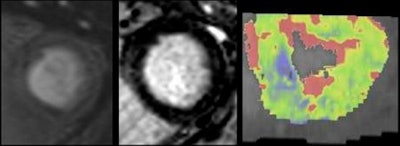 In a 65-year-old man presenting with angina, CT perfusion (right) shows ischemia in the inferolateral wall of the myocardium, but no infarction. CT perfusion also shows myocardial blood flow (MBF) reduced while myocardial blood volume (MBV) is well-preserved. All images courtesy of Dr. Konstantin Nikolaou.
In a 65-year-old man presenting with angina, CT perfusion (right) shows ischemia in the inferolateral wall of the myocardium, but no infarction. CT perfusion also shows myocardial blood flow (MBF) reduced while myocardial blood volume (MBV) is well-preserved. All images courtesy of Dr. Konstantin Nikolaou.In morphological imaging of the coronaries, it's all about the functional relevance of a stenosis -- and whether dynamic CT of the coronaries can provide the answers that will determine patient management. But first investigators must determine what the CT images mean, and whether the information is reliable.
"What is hypodensity on CT anyway?" Nikolaou asked. "Is it ischemia, is it infarction, is it scarring? Can we answer the question with a single perfusion dynamic scan or do we have to add a rest scan or a late enhancement scan?"
The mountain of questions is best scaled step by step, he said, and the next step is comparing dynamic stress perfusion CT to MRI to differentiate ischemia from viable myocardium. Can CT do it reliably? Maybe.
"Adding functional information in a time-resolved fashion with CT perfusion comparing ischemic versus nonischemic myocardium and showing this on color-coded maps answers the question, of course, and would be perfect to deliver in cases where you're not so sure," he said.
Early studies suggest the feasibility of assessment of myocardial perfusion using first-pass contrast patterns using single-shot acquisitions and dynamic acquisitions, he said. If proved accurate, such a technique could have a major impact on patient management.
The group enrolled patients with known or suspected coronary artery disease, ages 50 to 80, who were scheduled for invasive angiography. After injection with iodinated contrast, they underwent coronary CT angiography. This was followed by CT dynamic perfusion scans after injection of adenosine (140 µg/kg) on a dual-source scanner (Definition FLASH, Siemens Healthcare) acquiring 14 to 15 perfusion datasets with coverage of 73 mm.
"Keep in mind that acquiring 14 to 15 datasets limits the temporal resolution a little bit, and that influences the quantification of perfusion parameters -- so looking at these values plus the limited coverage of 70 mm, I would like to have a little more horsepower in terms of temporal resolution and scanner coverage in the next generation of scanners," Nikolaou said.
"Newer scanner generations might bring two significant advances: broader detectors for a reliable and sufficient coverage of the complete myocardium (for the dynamic type of the acquisition), as well as stronger tubes, allowing for higher tube currents and lower tube voltages at the same amount of image noise, i.e., with a maintained image quality," he explained in an email. "Using lower tube voltages will increase iodine absorption and will probably enhance depiction of ischemic versus nonischemic myocardium."
For quantitative analysis, maximum blood flow (MBF) and maximum blood volume (MBV) were acquired using parametric derivation.
CMR was acquired on a 3-tesla system (Magnetom Verio, Siemens Healthcare) with a 32-channel coil. Adenosine-induced hyperemia (140 µg/kg/min) and gadolinium were used to acquire steady-state free-precession (SSFP) images, rest and stress perfusion images, and late-enhancement images.
Statistical analysis included repeat analysis of variance (ANOVA) measures to determine differences between ischemic and nonischemic territories, maximum blood volume cutoff point derived by optimization of area under the curve (AUC, with two-sided asymptotic z-test), and conventional measures of diagnostic accuracy, Nikolaou said.
Of the 127 patients who met the inclusion protocol, 51 were protocol-eligible, and just 31 patients (mean age 61.7 ± 9, 18 men) ended up completing both the CT and MRI exams. "You know how these things go," Nikolaou said.
The average heart rate was 67.1 ± 9 bpm during coronary CT angiography (CCTA) and 83.1±16 during adenosine stress CT (85.0±10 at stress MRI). The effective radiation doses were 3.1 ± 1 mSv for CCTA and 10.0 ± 2 during CT myocardial perfusion imaging.
The prevalence of ischemic myocardial changes in the cohort was 28.4% ischemic segments and 13.3% infarcted segments.
"Myocardial blood flow was able to discern ischemic from normal segments -- just say any hypoperfusion versus normal myocardium comparing quant values," Nikolaou said.
The best threshold to evaluate the presence of any hypoperfusion was 88 mL/100 mL/min (c-statistics: 0.751, p < 0.001), which means a discrimination accuracy of 0.745 AUC for determining ischemic versus nonischemic, which is good but certainly not perfect, he noted.
Maximum blood flow was lower in ischemic/infarcted segments compared with normal segments: 73.2 ± 26 versus 104.8 ± 34 mL/100 mL/min, p < 0.001.
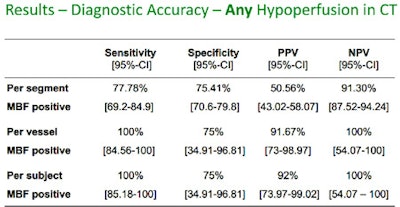 Results in 31 patients show that perfusion CT is moderately reliable for distinguishing ischemic from normal myocardium, and more sensitive for detecting infarcted myocardium.
Results in 31 patients show that perfusion CT is moderately reliable for distinguishing ischemic from normal myocardium, and more sensitive for detecting infarcted myocardium.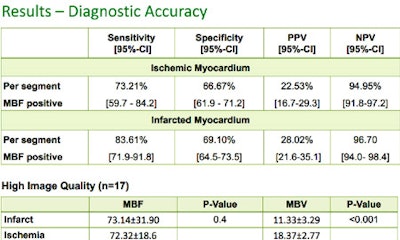
In terms of hypoperfusion, differences were seen depending on whether the analysis was based on segment, vessel, or subject, he said, with sensitivities ranging from 80% to 100% and specificities steady at 75%, and limited positive predictive values per segment.
"These data are quite good for a rather young technique to detect hypoperfusion," he said, and if you group the hypoperfusions into infarcted and ischemic myocardium at MRI, the CT data definitely show the difference between the two types.
CT did very well detecting infarcted myocardium at 85% sensitivity, but struggled a bit at 70% sensitivity for hypoperfused but viable myocardium, but, even so, sensitivity improved somewhat for transmural cases, he said. So the main limitation of CT was that "you tend to call areas hypoperfused when they are not," but "the sensitivity for infarcted and transmural perfusion defects and infarcted myocardium are, I think, quite satisfying."
The good news is that even today's scanner technology can assess perfusion defects with moderate diagnostic accuracy, although temporal resolution isn't quite high enough yet, Nikolaou said. It is a good start to see CT differentiating infarct from ischemia based on a single scan acquired with limited radiation dose and anatomic coverage.
"Of course, in the end, we'll have to prove that perfusion defects that we show have an impact on the clinical outcome in these patients," he said.
The group is currently focused on optimizing CT perfusion protocols to develop a reliable test, according to Nikolaou. For example, they are currently comparing dynamic, time-resolved CT myocardial perfusion with single-shot protocols, which are much more dose-efficient but do not provide time-resolved information, but rather an image showing iodine distribution at a single time point using single-shot techniques.
"In the end, I'm quite optimistic that this technique might be there in the clinical routine, but we'll have to find out together," he said.