Breast elastography can substantially improve ultrasound capability in differentiating benign from malignant breast lesions -- thus reducing the number of biopsies -- but which technique is the best? An Italian overview article in the European Journal of Radiology answers that question and more.
Mammography and ultrasound are often used in conjunction to characterize breast masses and assess the risk of malignancy. Ultrasound elastography has been introduced as a complementary modality for improving lesion characterization. Elastography may assess the stiffness of a lesion by mapping the strain in tissue elements subjected to an external compression, according to lead author Dr. Paolo Ricci, from the department of radiology, oncology, and pathology at Sapienza University in Rome, and colleagues (EJR, 2013 June 17).
Elastography has several technological options, and its role in clinical practice is not yet well-defined. Ricci and his team systematically reviewed recent literature on the diagnostic performance of breast ultrasound elastography in order to define its potential in differentiating benign and malignant breast masses.
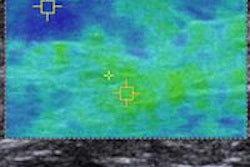
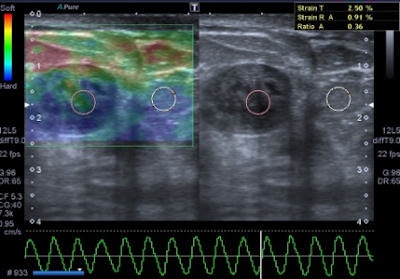
 A 47-year-old woman with a small breast lump. Baseline ultrasound shows a hypoisoechoic nodule with regular and well-defined margins. Elastography demonstrates a pattern 2 according to the Ueno-Itoh classification with prevalent elasticity, as a predominance of green, with few, if any, blue and red spots inside. Strain ratio, measured by placing two different regions of interest (placed in normal breast tissue and within the nodule), was 0.36. Gross pathology confirmed a benign fibroadenoma. All images courtesy of Dr. Paolo Ricci.
A 47-year-old woman with a small breast lump. Baseline ultrasound shows a hypoisoechoic nodule with regular and well-defined margins. Elastography demonstrates a pattern 2 according to the Ueno-Itoh classification with prevalent elasticity, as a predominance of green, with few, if any, blue and red spots inside. Strain ratio, measured by placing two different regions of interest (placed in normal breast tissue and within the nodule), was 0.36. Gross pathology confirmed a benign fibroadenoma. All images courtesy of Dr. Paolo Ricci.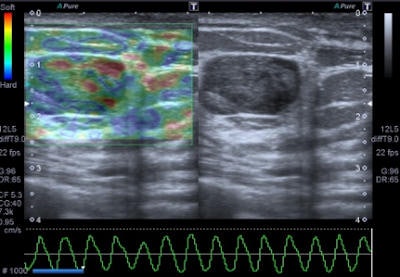
There are two different technical approaches: freehand elastography (USE) and shear-wave elastography (SWE), with the latter including acoustic radiation force impulse technology (ARFI) and supersonic shear-wave imaging (SSI), which also uses acoustic radiation force to push tissue remotely, but in a different way and with different steps. SWE is a trademarked name for SuperSonic Imagine's technology.
The techniques
Freehand ultrasound, or compression elastography ("strain elastography"), is based on the application of a compressive force to the breast and on the measurement of the shape-deforming effect, thus providing a value of lesion stiffness compared with that of surrounding tissues, the authors wrote. A slight manual compression/decompression is applied using a conventional transducer, or the deformation may be determined by respiratory movements.
"The technique allows only for qualitative and semiquantitative assessments of a lesion because the force exerted by manual compression is unknown to the equipment, thus allowing only the calculation of the deformability ratio (strain ratio) and not the absolute elasticity," Ricci wrote and colleagues.
The other type of technique, SWE, is possible because shear waves are ubiquitous and frequently occur in human soft tissues in which they are conducted by transverse displacement of the particles. Shear waves are transverse; they are rapidly attenuated by tissue, they travel much more slowly (between 1 and 10 m/sec), and they are not supported by liquids of low viscosity. Shear waves are produced by any mechanical disturbance and occur naturally from muscular movements (voluntary, cardiac, etc.), as well as being induced by the ultrasound systems used to measure their speed.
SWE comes in two different systems: a small measurement box (usually 5 x 10 mm) is set up within the tissue of interest and readings of the shear-wave speed are made, or a sensitive box provides real-time imaging (approximately 1 frame/sec) in a color-coded map of the shear-wave speed, allowing both qualitative and quantitative analysis.
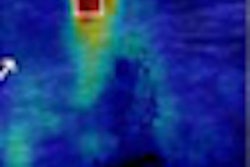
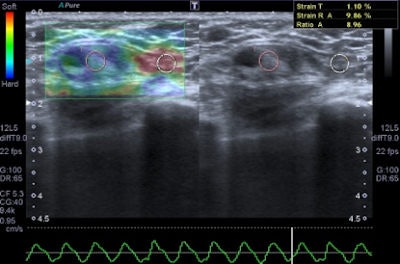
 A 45-year-old woman with a palpable mass in the lower-outer quadrant 2 cm away from the nipple. There was no erythema, ecchymosis, skin ulceration, or dimpling and no axillary lymphadenopathy was detected. Baseline ultrasound shows an isoechoic lump with regular margins, with some cystic areas within. At elastography the nodule presents a color map score 4 according to the Ueno-Itoh classification, with absence of elasticity, depicted as a completely blue area with a different peripheral glow. Placing two different regions of interest (in normal breast tissue and within the nodule), strain ratio obtained is 8.96. Gross pathology confirmed a cystic cancer.
A 45-year-old woman with a palpable mass in the lower-outer quadrant 2 cm away from the nipple. There was no erythema, ecchymosis, skin ulceration, or dimpling and no axillary lymphadenopathy was detected. Baseline ultrasound shows an isoechoic lump with regular margins, with some cystic areas within. At elastography the nodule presents a color map score 4 according to the Ueno-Itoh classification, with absence of elasticity, depicted as a completely blue area with a different peripheral glow. Placing two different regions of interest (in normal breast tissue and within the nodule), strain ratio obtained is 8.96. Gross pathology confirmed a cystic cancer.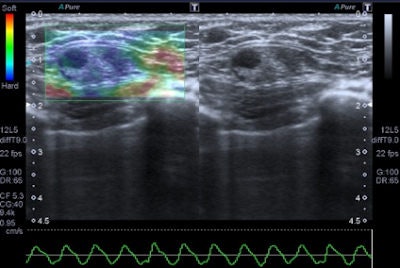
SWE is advantageous because the system "moves" the tissue by itself and no compression by the transducer is needed, and quantitative information (i.e., pressure wave velocity, maximum stiffness, average stiffness, etc.) is given, the authors wrote.
Two shear-wave technologies are available: ARFI and SSI. ARFI is a new sonographic elasticity imaging technology that noninvasively assesses tissue elastic hardness. SSI induces mechanical vibrations by using an acoustic radiation force created by focused ultrasound beam. A very fast (5,000 frames/sec) ultrasound acquisition sequence is used to capture the propagation of shear waves. This technology is available on a high-frequency probe basis for breast only, but SSI or SWE is available on six different probes to image several organs. The displacement induced in a tissue creates a shear wave that provides information about the local viscoelastic properties of the tissue, Ricci and colleagues noted.
Applications
Elastography can add important details to define the epithelial and connective tissue components and their different elasticity even if the technique appears more useful in the study of focal pathologies, the authors wrote. Focal benign disease usually appears as hypoechoic (solid nodular disease) or anechoic areas, more often with regular shape and clear boundaries. These nodules are usually round or ovoid in shape with a "wider-than-tall" appearance indicating an orientation that is parallel to the chest wall.
"Ultrasound structure is often homogeneous with a large variability of echogenicity. Vascularization is variable and dependent on nodule histology," they stated. "Generally, benign lesions are harder than normal breast tissue but softer than malignant tumors. Exceptions can occur and some benign lesions, such as hyalinized fibroadenoma and fat necrosis, can be hard at palpation and can cause false positives at elastography examination."
Benign lesions are described at elastography as lesions with a low color map score (score 1-2), while the best cut-off point for discriminating benign from malignant masses falling at the 3-4 boundary of color map classification, according to the Ueno-Itoh score system. Cysts often appear as hard and low deformable lesions, commonly represented with a blue pattern at the color map and with a high strain ratio because of their low compressibility.
The elastographic properties of fibroadenomas are controversial, because some studies report a substantial difference of fibrotic components than surrounding parenchyma, while other authors describe fibroadenomas as difficult to evaluate by color map because of similar elasticity to the breast gland, the authors noted. Strain ratio range reported by most authors for this specific type of lesion is around 2.1 ± 0.8. Fibroadenomas with larger fibrotic component and poor cellularity can have a suspicious color map, but in all cases the strain ratio is lower than malignant forms, they added.
Fat necrosis is usually seen as a hyperechoic stripe with inhomogeneous formation of fibrosis. The evolution toward sclerosis can cause images to be controversial for differential diagnosis. Radial sclerosing lesion causes architectural distortion of the gland with imposing fibrous reaction of the breast surrounding tissue that sometimes can degenerate or hide tumor features. Radial scars cannot easily be differentiated from malignant masses by a difference in elastography appearance in most cases, due to the higher strain compared with the healthy tissue around, they continued.
"While fat necrosis is reliably diagnosed using mammography, radial scars remain a diagnostic challenge that, ultimately, can only be verified as benign by means of pathology," they wrote.
Malignant lesions usually present with features such as acoustic shadow, spicules, indented margins, hyperechogenic halo with desmoplastic reaction around the lesion, calcifications, spreading along the ducts, and significant vascularization. Cancer can also have nonspecific features such as uneven architecture and gland edema.
Tumor stiffness is a characteristic of extracellular matrix, modulated by collagen cross-linking. Malignant lesions are less prone to deformation by pressure than normal breast tissue and have a more complex elastic modulus. Softer malignant lesions, including medullary, mucinous, papillary, cystic, and some necrotic infiltrating ductal carcinomas, are uncommon.
Elastography is often used in "uncertain" lesions classified as BI-RADS 3 and 4, but it does not change medical protocol in cases of BI-RADS 1, 2, and 5 lesions. The mean elasticity score of color map was significantly higher for malignant lesions than for benign lesions (p < 0.001), usually mainly represented as blue areas in the color map.
Limitations
Breast elastography has several limitations, mainly due to the structural aspects of the breast itself and the breast nodules, but also related to elastographic technique, according to the authors. The other limitations are listed below:
- The different expression of gland components within the population examined
- Absence of a capsule that allows containing compressing tissues
- Characteristics and high variability of lesions, often difficult to differentiate and with features of benignity or malignancy that may not match to their elasticity (cyst, necrosis, etc.)
- The uncertainty on reference parameters, particularly the need of adequate surrounded share of healthy parenchyma (vessels, fluid lesions including cysts, bone structures, etc.) that must be localized at the same depth of lesions to gain a right comparison
Adequate and standardized compressions also are important factors that may influence elasticity score, particularly in freehand examination, Ricci and colleagues noted.
"The general recommendation is that the transducer should be in very light contact with the skin at the beginning of examination because pressure and strain are no longer proportional beyond a certain degree of compression," the authors wrote. "In addition, both freehand ultrasound and shear-wave measurement approaches are associated with intra- and interobserver variability with regard to performing and interpreting elastosonographic data."
There is no consensus with regard to the best technique or classification to define elastography. As an ultrasound technique, elastography is influenced by operator dependence, especially because it requires some experience to obtain reproducible results.
Ricci and colleagues have some qualms with the current scientific literature: Overall, radiologists performing studies were not blinded to the clinical information.
"The results in current literature suggested that a better standardization is mandatory, mainly for compressive elastography," they wrote. "For example, the variability between experienced and inexperienced operators may limit reliability and diffusion of the method."
More studies are required because none of the studies reported patient-specific lesion depth from breast surface, lesion size, and palpability. Even evaluation to quantify typical static compressions' influence on diagnostic value of the mode in the case of shear waves assessment is deemed necessary because a failure to capture shear wave may occur, they wrote.
Finally, only a few studies described correlation between histological features and elastographic patterns, so the diagnostic and clinical performance of elastography in different kinds of lesions is not completely known to date.
Future developments
"In the current literature, various studies suggest an ancillary role of elastography in the differentiation of benign and malignant breast masses of any size," the authors wrote. "In fact, the common suggestion is to start investigation with high-quality conventional breast B-mode ultrasound and to apply then elastography if clinically needed. Moreover, some studies attest to the value of strain elastography in improving accuracy of the BI-RADS score."
Elastography can particularly help in upgrading a mass of low suspicion that would otherwise be sent for biopsy (BI-RADS 3) or downgrading a lesion classified as BI-RADS 4A, increasing confidence with its higher specificity and positive likelihood ratio when the findings concur with standard ultrasound findings.
"Rigorously designed and adequately powered studies are clearly needed to evaluate the diagnostic performance of the combination of ultrasound and elastography, to determine the most appropriate cut-off point of strain or velocity in different systems, and finally to assess the test performance across different lesion sizes and depths from breast surface," the authors concluded.
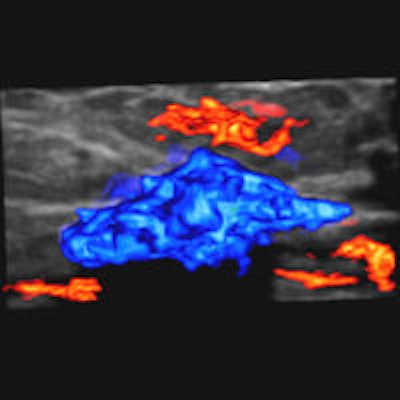
Editor's note: In the image on our home page, the shape and infiltration of the breast tumor is demonstrated in 4D Hitachi real-time tissue elastography (HI-RTE). Image courtesy of Hitachi Aloka, previously published in the ECR Today newspaper.