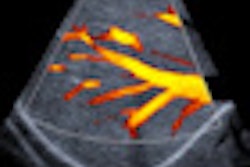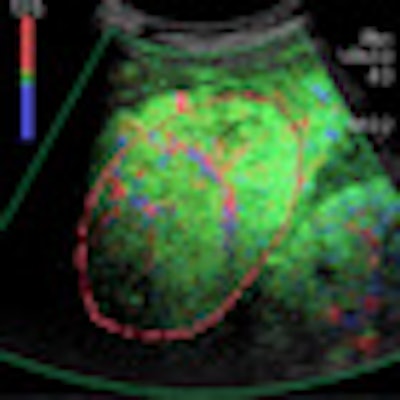
VIENNA - Contrast-enhanced ultrasound (CEUS) can help to characterize liver disease, but it is tricky to know how best to proceed when ultrasound delivers very different results to those of CT or MR. Experts shared their knowledge of this topic at the World Federation for Ultrasound in Medicine and Biology (WFUMB) congress.
Ultrasound contrast agents have worldwide approval for liver mass characterization, and the modality can match CT and MR in noninvasive diagnosis of focal liver disease because of contrast enhancement, according to Dr. Stephanie Wilson,a clinical professor of radiology at the University of Calgary in Canada.
There is generally widespread agreement between contrast-enhanced CT, MR, and ultrasound, especially in the arterial phase (AP). Very similar enhancement patterns also appear in the portal venous phase (PVP) during washout, which has a highly predictive value in malignant diagnosis. Displaying peripheral nodular enhancement (PNE) in hemangioma, the concordance between CT, MR, and ultrasound is as high as 93%, and the centripetal progression of enhancement for hemangioma has an equally high level of agreement.
A major source of disagreement, however, is timing discordance in the AP, as shown in a patient with primary colon cancer with a suspicious mass in the liver, she noted. With CEUS, four seconds following completion of AP flush, the mass was clearly hypervascular, whereas at 10 seconds, the lesion is already washing out, and at 18 seconds the liver is enhanced and the lesion has completely washed out. On CT, both in the AP and PVP, this lesion is "just" a low attenuation mass lesion.
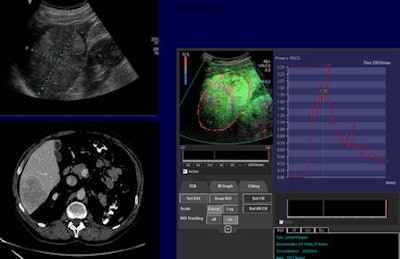 Contrast-enhanced ultrasound can play an important role in monitoring tumor response. This patient with hepatic metastasis of renal cell carcinoma was treated with temsirolimus (Torisel) and bevacizumab (Avastin). Image courtesy of Dr. Nathalie Lassau, head of ultrasonography unit at Institut Gustave Roussy in Villejuif, France.
Contrast-enhanced ultrasound can play an important role in monitoring tumor response. This patient with hepatic metastasis of renal cell carcinoma was treated with temsirolimus (Torisel) and bevacizumab (Avastin). Image courtesy of Dr. Nathalie Lassau, head of ultrasonography unit at Institut Gustave Roussy in Villejuif, France."If we plot the enhancement of the liver and the metastasis, we can see that the metastatic lesion enhances very rapidly, and by the time the liver enhancement has come up almost to its peak, the metastasis drops below it," Wilson explained. "If we consider that the CT AP images were taken in this time frame, it is no surprise that we miss that transient hypervascularity, which occurs in virtually all lesions. This timing discordance is something we anticipate seeing in patients with liver metastatic disease."
Timing discordance is also important for rapidly filling hemangiomas, where the pattern of enhancement is shown on dynamic real-time ultrasound, but not on snapshot in time CT scans.
In focal nodular hyperplasia, CT and ultrasound are concordant, but CT does not show the stellate vascularity and centrifugal filling provided by maximum intensity projection imaging, Wilson said. This discordance in vessel morphology can be particularly important for the differentiation of hypervascular masses in young asymptomatic patients.
Contrast agents may also increase disagreement, as shown in the case of a 26-year-old patient with fructose intolerance. Both CT and MR showed increased enhancement in the PVP, whereas CEUS showed a heterogenous hypervascular mass and a brisk washout. Discordance here was related to the different mechanisms of contrast agents.
"Being aware of this discordance is particularly important in cholangiocarcinoma and metastases," she pointed out, adding that fat in the lesion or the liver can also create disagreement, and pulse inversion scanning may not suppress a fatty liver. Here, MRI may prove both more sensitive and specific.
In focal liver lesions, the most common task is to determine malignancy, explained Dr. Christoph Dietrich, a professor of internal medicine at Caritas Hospital Bad Mergentheim in Germany.
"We have to assess whether it is a malignant or benign tumor by analyzing the iso- and hypoenhancing effect in the late phase. It has to be proven by biopsy in most circumstances because it can be, or it is probably, malignant," he said. "We have almost no malignant lesions, which show no hypoenhancement in the later phase, except neuroendocrine tumors in the liver cirrhosis, which might show hypoenhancement after three, four, five, or six minutes."
Dietrich listed the following practical limitations of conventional B-mode ultrasound:
Although often readily shown, very small metastases (< 5-10 mm) may be overlooked as they may be too small to produce visible defects in the portal and late phases.
Subdiaphragmatic lesions, especially those in segment 8, may not be accessible to conventional or CEUS. Intercostal scanning and positioning the patient in the left decubitus position can help reduce this limitation.
Because CEUS has limited penetration, especially in the case of hepatic steatosis or cirrhosis, deep-sited lesions may not be assessed. Scanning in the left lateral decubitus position brings the liver forward and closer to the transducer, and can help overcome this limitation and should be part of the routine survey.
The falciform ligament and surrounding fat can cause an enhancement defect that may be confused with a metastasis.
A potential pitfall is that small cysts, which were undetected on unenhanced ultrasound, are sometimes detected on late-phase scanning. These can often be distinguished from metastases because they characteristically show increased through transmission on CEUS. Careful re-evaluation with conventional ultrasound is necessary, he concluded.