Advanced radiotherapy delivery techniques have improved accuracy of treatment for many types of cancers but they require innovative dosimetry. Robust new quality assurance (QA) methods still need to be developed to verify these advanced dose distributions.
This was the subject of a dedicated session examining some of the options of novel dosimetry methods at the European Society for Therapeutic Radiology and Oncology (ESTRO) annual meeting in London.
Michael Duchateau, a doctoral candidate in translational radiation physics specializing in adaptive radiotherapy research at the Oncology Centre of the Universitair Ziekenhuis Brussel in Belgium, discussed some of the tools available for dosimetry of advanced dose distributions. He began his discussion with an announcement of good news and bad news about radiotherapy centers in Europe.
The good news: Radiotherapy centers achieve a compliance rate of nearly 98%. As an example of this, he said when a therapy treatment plan required the delivery of 1 Gy of radiation dose, 98% of the institutions delivered precisely 1 Gy.
The bad news: In a recent audit, 30% of credentialed institutions failed to accurately deliver an intensity-modulated radiotherapy (IMRT) head-and-neck dose distribution. "New technologies are error-prone; IMRT is still not plug-and-play," Duchateau explained. "It's a struggle for centers to reach the comfort zone and do better at the next credentialing."
Dosimetry selection
One newer option available for quality assurance of 3D dose distributions is an array of either ionization chambers or diodes. Such arrays can have a linear design or employ a rotational geometry. Linear arrays can be used for both field-by-field and composite dosimetry, and can perform machine quality assurance. The downside is their significant angular dependence, Duchateau explained.
Rotational arrays that are specifically designed for quality assurance of arc therapies and tomotherapy enable fast composite QA. As examples, Duchateau referenced some of the newer commercial systems for rotational dosimetry, such as ArcCheck (Sun Nuclear, Melbourne, FL, U.S.) and Delta4 (ScandiDos, Uppsala, Sweden).
"Arrays offer a good alternative to film because you can do processing on the fly and lots of add-ons are available," he said. "The big point to remember is 'try before you buy,' because many different arrays are available and you have to validate these before use in clinics. You have to QA the QA tools that you're going to use."
Another method employed for QA of advanced radiotherapies is dose reconstruction. This can be performed with built-in electronic portal imaging devices, in which grayscale images are converted to dose, or by using add-on options such as Compass (Ion Beam Applications, Louvain-la-Neuve, Belgium) or 3DVH (Sun Nuclear).
Benefits of dose reconstruction include the fact it is patient-specific and users can define their own workflow. One downside is the large amount of data generated, which requires good information and communication technology resources. Duchateau noted, however, this method does not provide physical 3D dose measurement, only 3D reconstruction and the accuracy is based on a calibration or simulation model.
He also described the emergence of chemical gel dosimeters, based on monomers that polymerize upon exposure to radiation or radiochromic gels that change color upon irradiation. "No other dosimeter can serve as a 3D dosimeter today," Duchateau said. "It's real 3D dosimetry that can be used for end-to-end testing."
The process of gel dosimetry is, however, relatively complex, involving the creation of a gel phantom, irradiation, and quantitative imaging (mostly via MR or optical CT) of the resulting 3D dose distribution inside the gel. The overall accuracy is dependent upon the gel properties, as well as the readout technique used. Research is now focused upon improving usability, as well as minimizing toxicity.
In conclusion, Duchateau rhetorically asked whether QA tools are keeping up with the rapid evolutions in treatment modalities. Noting that it's still difficult to reach the comfort zone for quality assurance of techniques such as IMRT, he suggested that they are not.
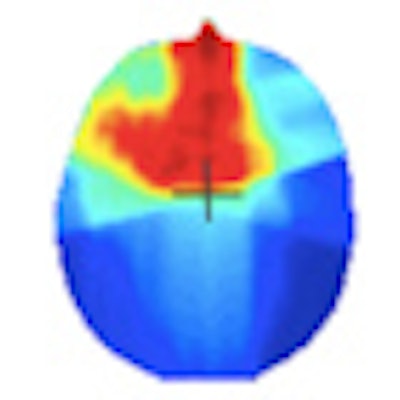
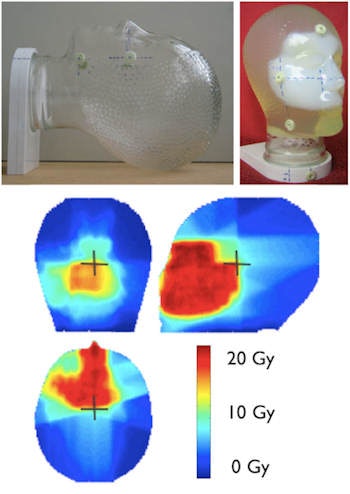 Gel dosimeters enable true 3D dosimetry. Image courtesy of Yves De Deene from Ghent University.
Gel dosimeters enable true 3D dosimetry. Image courtesy of Yves De Deene from Ghent University.Optical options
Next, Claus Andersen, of Risø DTU at the Technical University of Denmark in Roskilde, told the ESTRO delegates about the use of online luminescence dosimetry for monitoring radiation therapy. Luminescence dosimetry is performed using an optical fiber-based probe containing a small amount of organic plastic scintillator or inorganic crystalline phosphor. During irradiation, the scintillator spontaneously generates radioluminescence in proportion to the instantaneous dose rate.
As the millimeter-sized probe contains no electrical wires or other electronics, it can be used for in vivo dosimetry. The generated optical signal is recorded in real-time via the optical cable, which can be connected to remote readout devices.
As well as producing radioluminescence, some crystalline inorganic phosphors (such as carbon-doped aluminum oxide, Al2O3:C) also trap energy. After irradiation, this stored energy is released as luminescence by exposure to laser light, creating an optically stimulated luminescence signal that provides a measure of accumulated dose. After each use, the crystal can be "reset" via further laser treatment. Andersen noted, however, that this process can take five to 10 minutes, which could be problematic in a clinical setting.
Andersen also described some of the challenges associated with online luminescence dosimetry. First, there's ionization quenching, which implies the amount of light produced per absorbed dose unit is not the same in, for example, kV and MV x-ray beams.
The "stem effect," in which irradiation of the optical fiber itself produces light, also needs to be addressed. For inorganic phosphors, this can be achieved by exploiting the long lifetime of luminescence to separate it from the stem signal produced in pulsed linear accelerator beams. This approach cannot be used with the fast organic scintillators though, and other solutions such as chromatic separation are necessary. Andersen noted, however, that such plastic scintillators are of particular interest due to their direct water equivalence.
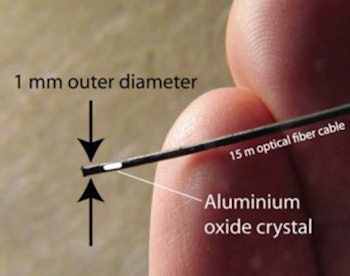 The fiber-based dosimetry probe contains a small amount of organic plastic scintillator or inorganic crystalline phosphor, such as carbon-doped aluminum oxide.
The fiber-based dosimetry probe contains a small amount of organic plastic scintillator or inorganic crystalline phosphor, such as carbon-doped aluminum oxide.Promising applications for the probes include point measurements for in vivo dosimetry, as well as reference dosimetry in small fields. For brachytherapy dosimetry, for example, probes can be placed in unused channels of a standard brachytherapy applicator to perform in-tumor dosimetry. Another example is the use of a thin layer of Al2O3:C on the fiber for particle therapy dosimetry.
Andersen concluded by emphasizing that online luminescence dosimetry is an emerging technology. When questioned as to why the method has not yet been commercialized, Andersen suggested the use of delicate fiber cables within clinics may be an issue.
"We need more demonstrations showing that it works in small studies and then we'll move on," he said.
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.



















