DICOM CDs are commonly used today to disseminate patient images and information, but many of them suffer from deficiencies that can hinder image viewing and integration into PACS networks. To help deal with the problem, the German Roentgen Society (DRG) initiated a quality assurance initiative designed to improve the quality of DICOM CDs.
Definitions exist in DICOM Part 10 and the Integrating the Healthcare Enterprise (IHE) portable date interchange (PDI) profile for CDs, but their usability has been restricted by organizational and technical problems, according to Dr. Peter Mildenberger of the University of Mainz in Germany. He discussed the DRG's approach to improving the situation during a session at the 2006 RSNA meeting in Chicago.
Problems with DICOM CDs today include machine readability issues (such as defective media), workflow integration problems, and limited quality of viewers, he said. In addition, the CDs are unreadable 5% to 10% of the time, he said.
In 2005, the DRG launched an initiative in collaboration with the Oldenburg Research and Development Institute for Information Technology Tools and Systems (OFFIS) in Oldenburg, Germany, to develop product specifications, recommendations for users, and testing procedures for products.
While standards are available today, they are often incorrectly or incompletely implemented, Mildenberger said. In addition, many small companies may be unfamiliar with the standards, and support for IHE PDI is only available in some offerings, he said.
The initiative resulted in vendor specifications for DICOM CDs, based on DICOM Part 10 and IHE PDI.
"Maybe 95% of the recommendations are already covered by IHE PDI," he said. "We needed some national extensions -- for example, Cardio-profile."
The initiative also produced recommendations for users, Mildenberger said. In addition, testing tools were developed and procedures were set up for technical and practical testing.
Designed to facilitate "plug and play" interchange of radiological reports, the specification of a best practice DICOM CD is based on the IHE PDI specification with some extensions, he said.
Among the specification's principles: medical images in DICOM format are required. Other non-DICOM content such as reports and discharge letters are allowed, though. A DICOM viewer application or IHE "Web content" is allowed but optional, Mildenberger said.
Vendors are encouraged to adapt their products to the requirements specification, which is available in German at this Web site.
Storage medium and file system requirements include the use of CD/R and CD/RW media with an ISO 9660 file system and no packet writing, according to Mildenberger. The creator must also ensure the absence of malicious software such as viruses and spyware. Labeling of the CD is required, containing the patient's name, birth date, the CD's creator, date of study and CD creation, and type of content.
If present, DICOM viewing software must be able to correctly display all DICOM content of the CD. The initiative recommended inclusion of a PDF manual and a printed short manual with the CD. An "autostart" viewer is not recommended.
At the 2006 German Congress of Radiology, users were invited to submit DICOM CDs for a short live test during the show. Designed to provide an overview of the state of the art in the market, the test also served as a good trial run for the initial prototype of the conformance test suite, according to Mildenberger.
Overall, 65 CDs representing 44 different products and 27 different vendors were tested.
The testing procedure included automated test software for the type of file system, directory structure, absence of malware, and conformance of DICOMDIR. A manual test of the DICOM viewer and Web content, if available, was also performed. CD labels and manuals were also visually inspected, if present.
Of the submitted CDs, 74% had violations of the specifications and 5% were defective or had no DICOM content.
"Almost 80% of the real-world CD's failed," Mildenberger said. "This clearly shows that despite all of the activities of DICOM and IHE, a quality assurance program is needed."
Typical DICOM errors included violation of DICOM rules for file names and directory names. "This is a beginner's mistake for DICOM programmers," he said.
Other errors included DICOMDIR fields that were missing or empty, violations of syntax rules for DICOM data types, and incorrect transfer syntax for DICOM images, according to Mildenberger.
Typical problems with the DICOM viewer included requirements for administrator privileges or failure to run at all. Often, no documentation or manual was available. In addition, CD labeling was often missing and almost always incomplete, he said.
"Most of these problems would be easy to avoid for the software vendor," Mildenberger said.
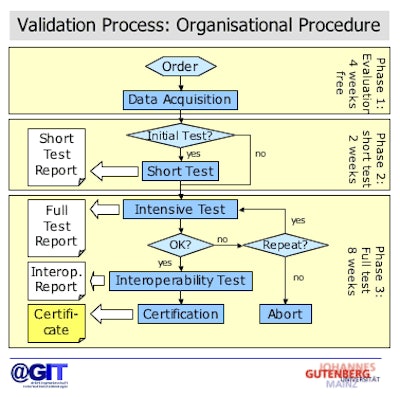
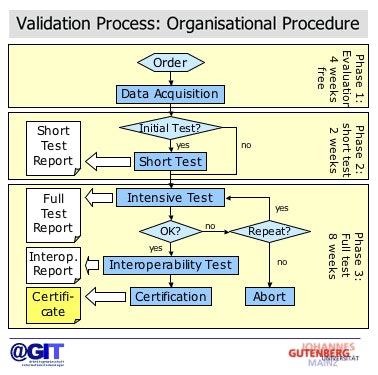 |
| Validation process for DICOM CDs. Image courtesy of Dr. Marco Eichelberg (OFFIS) and Dr. Peter Mildenberger. |
Since the testing process, an improvement has been seen in the marketplace, he said.
"Companies have learned from their mistakes and have improved their products (very fast)," Mildenberger said. "It is our expectation that users will ask for this testing process, which is implemented now."
By Erik L. Ridley
AuntMinnie.com staff writer
January 22, 2007
Related Reading
IHE PCC committee releases technical framework, August 22, 2006
Registration for IHE Connectathon 2007 begins, August 21, 2006
IHE's RFD supplement opens for comment, August 15, 2006
IHE's Import Reconciliation Workflow eases portable media handling, July 17, 2006
Copyright © 2007 AuntMinnie.com
















