Molecular radiotherapy uses radiopharmaceuticals to deliver radiation directly to malignant cells, whilst minimizing damage to healthy tissue. The technique employs a range of targeting mechanisms -- including metabolic processes, cell surface receptors, extracellular targeting, and direct injection into the tumor -- to direct the radiopharmaceuticals to molecular sites and receptors.
Also known as targeted radionuclide therapy or internal radiotherapy, molecular radiotherapy has been used to treat cancer for many decades. Example applications currently in clinical use include I-131 for treatment of thyroid cancer, Lu-177, Y-90, and I-131 for treating neuroendocrine tumors and Ra-223, among others, for palliative therapy of bone metastases.
"There are many different isotopes -- auger emitters, alpha emitters, beta emitters -- that are used for many different cancers," explained Ana Denis-Bacelar, PhD, a research scientist in nuclear medicine physics at the Institute of Cancer Research and the Royal Marsden National Health Service (NHS) Foundation Trust in London. "The radiobiological effects of all of these treatments are quite different. This makes the field challenging, and exciting."
Dosimetric challenges
One major challenge for molecular radiotherapy is the lack of established procedures for calculating the absorbed dose to the target. Speaking at the recent meeting of the Medical Physics Group of the U.K. Institute of Physics, Up and Coming Techniques in Medical Physics Translated into Clinical Practice, Denis-Bacelar explained that molecular radiotherapy is not planned according to the radiation dose delivered. Instead, most treatments are based on fixed levels of administered activity -- sometimes modified according to the patient's weight.
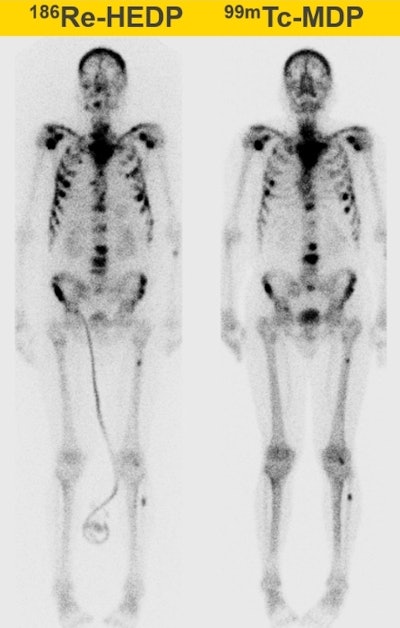 Whole-body therapeutic (Re-186 HEDP) and diagnostic (Tc-99m) bone scans of a patient with bone metastases. All images courtesy of Ana Denis-Bacelar.
Whole-body therapeutic (Re-186 HEDP) and diagnostic (Tc-99m) bone scans of a patient with bone metastases. All images courtesy of Ana Denis-Bacelar.But physiology differs from patient to patient, and as a result, the absorbed dose can vary by up to two orders of magnitude. "Dosimetry is not mandatory and is not usually performed, because it hasn't been proved to be useful in large randomized clinical trials. But we can't prove that it is useful if we don't do it," she pointed out.
So why is dosimetry-based molecular radiotherapy not routine? One reason, Denis-Bacelar said, lies in the success of radioiodine treatments, which have been used to treat thyroid cancer for some 75 years. The good success rate seen when administering a standard fixed activity has led users to surmise that dosimetry is simply not required.
Another confounding issue is that calculation of the absorbed doses from internally delivered radiation is not straightforward. Unlike external-beam radiotherapy, where delivered dose depends upon the radiation characteristics and the alignment of the patient with the beam, and dosimetry is highly accurate, in molecular radiotherapy, the delivered dose depends upon the biokinetics of radiopharmaceutical as well as the physiology of the patient. As a result, dosimetry of molecular radiotherapy is patient-specific and difficult to predict.
So how can this shortfall be addressed? One attempt lies in the European Directive 2013/59/Euratom, which mandates that personalized dosimetry-based treatment planning is put in place by February 2018. Whether this will be adhered to or even achievable remains to be seen.
But despite the complexity of the task, research efforts are underway to implement internal dosimetry, using techniques such as 3D-image-based dosimetry. The radionuclide dose absorbed by a target can be defined as the cumulated activity (which is patient-specific and dependent upon biokinetics and uptake distribution) multiplied by a dose factor (a physical characteristic of the radionuclide). The activity can be determined using 360° SPECT, for example, to image the patient after radiopharmaceutical administration.
SPECT can provide quantitative information -- but the images must be highly accurate in order to convert counts into activity. Challenges here include scatter in the patient, which can be addressed using triple-energy window and Monte Carlo-based scatter correction methods, and attenuation, where incorporation of CT images can help. Other tasks to consider include image reconstruction and registration (where motion may impact accuracy), as well as dealing with factors such as collimator detector response, low spatial resolution, and dead time.
Clinical cases
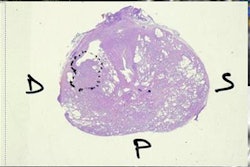
Denis-Bacelar presented some example studies investigating dosimetry of bone palliation treatments with Ra-223, which is currently the fastest growing application of molecular radiotherapy (according to a recent Internal Dosimetry Users Group survey). Bone metastases cause pain, fractures, and a general decrease in the patient's quality-of-life, and are most commonly treated palliatively. Molecular radiotherapy is usually delivered based on a fixed Ra-223 activity, sometimes adjusted for the patient's weight.
One study, for instance, examined Re-186 HEDP treatments of bone metastases from prostate cancer, where the dose-limiting factor is blood marrow toxicity. The researchers created a model to estimate the whole-body absorbed dose for an individual patient before radionuclide injection, using the level of administered activity and preinjection biochemical measurements. Results revealed the whole-body absorbed dose was correlated with blood toxicity. "This showed that we can predict the toxicity prior to treatment and thus adjust and personalize it," Denis-Bacelar explained.
 A transversal slice through the pelvis of a SPECT-based absorbed dose distribution of a patient with bone metastases treated with Re-186 HEDP.
A transversal slice through the pelvis of a SPECT-based absorbed dose distribution of a patient with bone metastases treated with Re-186 HEDP.She also described her recent work on image-based dosimetry of Re-186 HEDP treatments (European Journal of Nuclear Medicine and Molecular Imaging). The study calculated SPECT-based absorbed doses for 379 bone lesions in 22 patients treated with 5 Gbq. Results revealed a wide range of absorbed dose, both between lesions in one patient and between patients, even though all received the same activity.
The researchers also investigated the relationship between absorbed dose and overall survival, and found that increased dose actually correlated with poorer survival. However, Denis-Bacelar explained that disease volume and absorbed dose are correlated, and that patient stratification according to disease is required in order to effectively study dose-response relationships.
The next logical step may be to transition from using molecular radiotherapy for pain palliation to actually treating bone metastases, with the aim of prolonged survival. Denis-Bacelar and colleagues have developed a radiobiological model to predict the reduction in metastatic burden as a function of the absorbed dose delivered from molecular radiotherapy. In a study of 22 patients, the model predicted that a small increase in absorbed dose could lead to a large tumor reduction in most patients. "This tool may provide a way to select patients suitable for this therapy," she noted.
Looking ahead
Denis-Bacelar rounded off her presentation with a look at what's coming next. Molecular radiotherapy is growing, she said, and new commercial radiotherapeutics -- such as Ra-233-based drugs, Y-90 microspheres, Lu-177 DOTATATE, and Y-90 DOTATOC -- are being introduced. These new drugs may be very expensive, however, making standardization of imaging and dosimetry protocols essential. "We are getting there slowly," she said.
Elsewhere, modeling of the imaging system and 3D printing of more realistic tumor/patient phantoms will help improve imaging-based dosimetry. Emerging detector technologies -- such as those based on CZT (cadmium zinc telluride), which offers improved scatter correction and high count-rate operation -- should also help.
In conclusion, Denis-Bacelar emphasized that presently, most molecular radiotherapy treatments are not planned according to absorbed dose but are still based on a fixed activity -- and one size does not fit all. The incorporation of imaging will enable personalized treatment planning, with image quantification, though challenging, essential for accurate dosimetry.
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.