To mark the 60th anniversary of Physics in Medicine and Biology (PMB), the journal hosted a symposium at Imperial College, London, as part of the 18th International Conference on the Use of Computers in Radiation Therapy (ICCR 2016). The symposium attracted nearly 300 delegates from around the world and focused on the evolution of medical physics.
Of all the speakers, Robert Jeraj, PhD, from the University of Wisconsin in Madison, faced the hardest task: predicting the future. For a physicist, this usually involves examining current trends and extrapolating. But the future is not always clear, "even 10 years ago, people did not predict some discoveries like immunotherapy," he noted.
Jeraj suggested that medical physics is one in spirit with medicine, and may progress along a similar path. He cited the "four P's of medicine": prediction of an individual's disease development; personalized treatment; pre-empting disease before it occurs; and participation of patients, communities, and healthcare providers. This rationale gives rise to the idea of precision medicine, which addresses the highly heterogeneous nature of cancer. In the past, little could be done to tackle this, but the emergence of targeted therapies is bringing personalized therapies within reach.
Unfortunately, there are obstacles to overcome. For example, cancers evolve in time to become harder targets to treat. So how can medical physics help? "We need to capture the whole disease and the evolution of disease, using multimodality imaging techniques such as optical and molecular imaging," Jeraj explained. In radiation therapy, such a shift from anatomical imaging and population-based treatment, to molecular imaging, patient-specific therapies and nonuniform dose delivery is the foundation of "biologically conformal radiotherapy."
Jeraj noted that medical physics must also strive to treat more advanced cancers. He described a case in which PET imaging of a patient with advanced metastatic prostate cancer revealed multiple, extremely heterogeneous lesion responses: responding, nonresponding, stable, emerging, and disappearing. "How can you treat this patient with one modality? You can't," he said. But such information would possibly enable selective localized targeting (by radiotherapy, for example) of selective nondrug-responsive lesions. "Radiotherapy can be exploited to big advantage of patients with highly advanced disease."
This idea of extracting large amounts of useful data from a single medical scan is the premise underlying radiomics -- a promising, emerging field. However, much more data are available from genomic and other tests. To analyze such large amounts of data, Jeraj said, we must learn from "big science" physics approaches. When analyzing data to detect the Higgs boson, for example, CERN physicists relied on a deep understanding of the underlying fundamental physics.
"Likewise, when we move to medicine, we have to understand the underlying biological principles that drive observations," Jeraj explained. Image analysis and modeling of disease require accurate knowledge of the biology. To achieve this effectively, it's essential that physicists partner with biologists. "These problems are way too hard to tackle alone," he said.
With medical physics perhaps on an inevitable path toward incorporating more and more biology, what could the next 60 years hold for Physics in Medicine and Biology? According to Jeraj, by then, the journal may require a name change -- to Physics and Biology in Medicine.
The medical physics revolution
Steven Meikle, PhD, from the University of Sydney described just how far medical physics has come in the last 60 years. Looking first at the current state-of-the-art in cancer diagnostics, he explained how this involves anatomical imaging with CT, ultrasound, or MRI, plus PET/CT scans for staging. Treatment planning can be based on CT, MRI, or PET/CT, as can posttreatment imaging to monitor effectiveness. In 1956, only planar x-ray imaging was available. This was used for diagnosis, but not routinely for planning, while posttherapy monitoring relied solely on clinical observation.
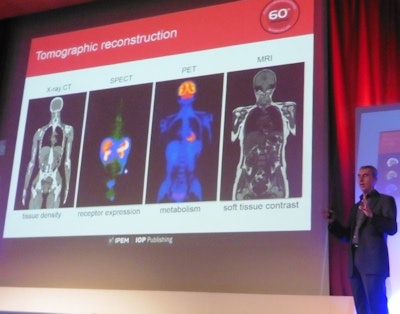 Steven Meikle, PhD, from the University of Sydney examines the major advances that enabled the "medical physics revolution." Credit: Tami Freeman
Steven Meikle, PhD, from the University of Sydney examines the major advances that enabled the "medical physics revolution." Credit: Tami FreemanFor radiotherapy, treatment planning in 2016 uses model-based or Monte Carlo methods. Radiation is delivered via conformal techniques such as intensity-modulated radiotherapy (IMRT), image-guided radiotherapy (IGRT), volumetric-modulated arc therapy (VMAT), and stereotactic body radiotherapy (SBRT), using x-rays, electrons, photons, or ions. Verification is performed after, or even during, treatment delivery. In 1956, plans were hand-calculated based on anatomical landmarks and delivered using kilovoltage x-rays or high-energy gamma rays.
"You can see by comparing workflows that imaging has made a huge contribution to the way we practice cancer diagnosis and staging, and medical physics has played a big part in that," Meikle explained. "Medical physics has also made a big contribution to the way external-beam radiotherapy is applied for cancer treatment."
Meikle examined the major advances that enabled this "medical physics revolution." "In imaging, I nominate tomographic reconstruction as the single biggest advance that moved the field forward," he declared. Indeed, the significance of this development was recognized back in 1979, when Allan Cormack and Godfrey Hounsfield won the Nobel Prize in Physiology or Medicine for their development of computer-assisted tomography.
In radiotherapy, major advances concern the emergence of conformal radiotherapy, including beam shaping with multileaf collimators in the 1980s, IMRT in the 1990s, and IGRT in the 2000s. "All of these progress radiotherapy treatment planning and delivery to allow much more conformal and precise dose distributions," he said.
Meikle concluded by considering how such advances impact patients. "Over the last 60 years, medical physics has helped to reduce cancer mortality and improve quality-of-life for cancer patients," he said. "Life expectancy is greater now than 60 years ago. But we are also losing more years to ill health and disability. It's easy to look back and think all the problems have been solved. But chronic illness is still a big problem -- and medical physicists are well equipped to help solve this problem."
Evolutions in diagnostic imaging
Brian Pogue, PhD, from Dartmouth College in Hanover, New Hampshire, discussed the evolution of diagnostic imaging over the last 60 years. He noted that between the 1990s and 2000s, PMB saw a jump in imaging publications per year, with citations in this field growing even faster.
 Brian Pogue, PhD, from Dartmouth discusses the evolution of diagnostic imaging. Credit: James Dacey
Brian Pogue, PhD, from Dartmouth discusses the evolution of diagnostic imaging. Credit: James DaceyPogue shared some example successes in medical imaging, such as low-dose CT, which was advanced by the development of new detectors, statistical modeling algorithms, iterative reconstruction, and patient-specific scans, as well as public awareness of need for low-dose scans. PET/CT and SPECT/CT, meanwhile, evolved thanks to hardware developments, improvements in reconstruction, and widespread adoption.
Another example is x-ray tomosynthesis for breast imaging, which was enabled by new detectors and sources, reconstruction algorithms, computer-aided detection (CAD) algorithms, and screening recommendations. Elsewhere, dual- and multienergy CT are now widely adopted, with the field in an exponential growth phase.
Then there's 3D ultrasound, which has benefited from faster digitization and 3D reconstruction techniques. "This might look like a technical curiosity, but its impact has been profound in both neonatal and breast cancer imaging," Pogue pointed out. Other success stories include MRI breast and prostate applications, surgical guidance and radiotherapy-positioning technologies, near-infrared spectroscopy, and robotic procedures.
Pogue also described the use of contrast agents to improve resolution, contrast, and function assessment, citing growth in the use of ultrasound microbubble contrast as one example. "We can use contrast agents to push imaging to its ultimate extent, by using superresolution methods with bubble localization to achieve micron-level spatial resolution across a centimeter of tissue," he added.
Finally, Pogue mentioned some fundamentally new physical imaging techniques that use multiple electromagnetic or acoustic spectral bands to image tissue with combined information streams. These included magnetic particle imaging, an embryonic field in a rapid growth phase, as well as photoacoustic imaging, bioluminescence, and Cerenkov imaging. "PMB has published some of the most important papers," he noted.
See what you treat
Bas Raaymakers, PhD, from UMC Utrecht in the Netherlands, considered the need for image guidance during radiation therapy. With advanced modalities such as IMRT allowing the sculpting of dose distribution, it becomes imperative that radiation is accurately delivered to the correct place. "Image guidance becomes more and more critical with these types of radiotherapy delivery," he explained.
 Bas Raaymakers, PhD, from UMC Utrecht considers the need for image guidance during radiation therapy. Credit: James Dacey
Bas Raaymakers, PhD, from UMC Utrecht considers the need for image guidance during radiation therapy. Credit: James DaceyChanges in patient and tumor anatomy during a fractionated radiation course necessitate imaging between fractions to ensure the treatment remains accurate. "If you're going to deliver radiation 30 times, things are going to change," Raaymakers said. And even during treatment, motion due to breathing, for instance, can create uncertainties in dose delivery. Image guidance can help mitigate such uncertainties.
Raaymakers described the wide range of options available for guidance of external-beam radiotherapy. In-room CT-on-rails systems offer imaging prior to, though not during, treatment. Megavoltage imaging with treatment beam can be used before or during treatment and provides good bony anatomy visualization, albeit with low contrast and signal-to-noise ratio. Fluoroscopy systems also offer real-time guidance during irradiation.
Ultrasound can now be employed for treatment guidance, with Elekta's Clarity allowing inter- and intrafraction imaging, and other systems offering interfraction ultrasound guidance. Other approaches include radiofrequency tracking of implanted beacons and on-board cone-beam CT. Further ahead, biological guidance may be possible, enabled by the PET-guided system under development by RefleXion Medical.
Ultimately, MRI may prove the optimal guidance modality, offering real-time visualization of the tumor and its surroundings. As such, several groups worldwide are developing MRI-guided radiotherapy systems. The MRIdian from ViewRay, for example, which is based on three cobalt-60 sources and a 0.35-tesla MRI, was first used to treat a patient at the start of 2014 and is now in regular clinical use.
The AuroraRT, developed at the University of Alberta in Canada, integrates a 6 MV linac with a 0.5-tesla magnet. The first prototype was operational in December 2008 and the system is now being commercialized by MagnetTx. The Australian MR-linac project is also developing an integrated system, producing an operational prototype in January.
Raaymakers and colleagues at UMC Utrecht, Elekta, and Philips have developed a high-field MRI-guided radiotherapy system that integrates a 1.5-tesla MRI with a 6 MV linac. The first prototype was built in 2009, and a clinical system is now under construction and being commercialized by Elekta under the name Atlantic.
"Image guidance is major research arena for radiotherapy, and MRI guidance offers great potential," Raaymakers concluded. "Sight during treatment enables better targeting, better sparing, and better dose-response assessment."
New horizons in adaptive therapy
Once it is possible to "see what you treat," the next step is to perform adaptive radiotherapy (ART). Katia Parodi, PhD, from Ludwig-Maximilians-Universität München in Germany examined the key ingredients of modern ART, explaining first how the use of graphics processing units (GPUs) to accelerate computation made a huge impact in this field.
 Katia Parodi, PhD, from Ludwig-Maximilians-Universität München discusses adaptive radiotherapy. Credit: James Dacey
Katia Parodi, PhD, from Ludwig-Maximilians-Universität München discusses adaptive radiotherapy. Credit: James DaceyGPUs can run accurate Monte Carlo-based VMAT dose calculations in less than 40 sec -- opening up the possibility of dose recalculation while the patient is on the table. "This could allow us to go towards plan-of-the-day adaptation," Parodi noted. Accelerated dose calculation also applies in particle therapy, where GPU-based Monte Carlo proton calculations have been achieved in less than 4 sec per million particles.
Rapid computation also enables the application of advanced planning strategies, such as multicriteria optimization (MCO), in which multiple plans are calculated and optimized. MCO is already deployed for photons and under development for ions.
Another critical ingredient is in vivo dose reconstruction. "If you can reconstruct the actual delivered dose, this could open a new way to document treatment," Parodi said. "And if you can do this fast enough, it may be even possible to correct the treatment if something is going wrong."
Parodi described some novel methods for in vivo dose reconstruction in photon therapy, including Cerenkov imaging and thermoacoustics. For particle therapy, she highlighted the recent renewed interest in ion-based transmission imaging for planning. Likewise, approaches such as PET, prompt gamma imaging, and ionoacoustics are under investigation for monitoring dose delivered during treatment.
Looking forward, Parodi suggests that we may now be entering the era of biological guidance. "The next dimension to explore for ART is biological guidance," she told the delegates. One example is the use of PET for stratification, therapy personalization, and response assessment. Including oxygen-enhancement ratio in ion beam planning, meanwhile, could enable dose or cell killing painting of inhomogeneous tumors.
Parodi concluded by presenting some emerging methods that exploit biology. These include micro- and minibeam therapies, spatially fractionated approaches designed to spare healthy tissue that have been demonstrated with both photon and proton beams. Another possibility is to use nanoparticles for tumor vascular disruption during radiotherapy.
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.