Siemens Healthineers is showcasing two new major product launches at ECR 2018. The introductions include the 1.5-tesla Magnetom Sola MRI scanner with the vendor's BioMatrix technology, and Acuson Juniper, a premium shared-services ultrasound scanner developed after an extensive customer survey effort.
New BioMatrix sensors
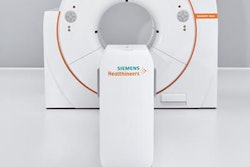
Magnetom Sola sports a 70-cm magnet bore and features an expansion of the company's BioMatrix technology for addressing unwanted patient-specific variations in MRI examinations. Siemens first introduced BioMatrix, which automatically adjusts MRI scanning parameters based on patient anatomy and physiology, on the vendor's Magnetom Vida 3-tesla scanner at ECR 2017. The launch of Magnetom Sola brings BioMatrix to a 1.5-tesla scanner.
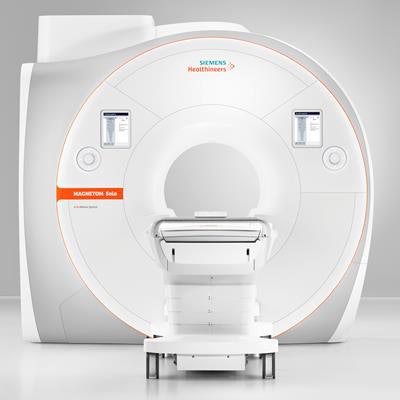
 Siemens' Magnetom Sola 1.5-tesla MRI scanner. Image courtesy of Siemens Healthineers.
Siemens' Magnetom Sola 1.5-tesla MRI scanner. Image courtesy of Siemens Healthineers.In addition to the BioMatrix Respiratory Sensor that was included on Magnetom Vida, Magnetom Sola benefits from two new BioMatrix sensors: Beat Sensor and Kinetic Sensor. Beat Sensor, which is integrated into the scanner's new body coil, is designed to automatically capture heart motion in real-time and help to trigger sequences based on the cardiac rhythm, according to Andreas Schenk, vice president of sales and marketing for the company's MR business line.
Similarly, Kinetic Sensor is an in-bore camera system that recognizes a patient's head motion in real-time, and then adjusts the MRI scan based on the real-time motion information. As a result, it yields a significant reduction in artifacts and higher diagnostic image quality, while avoiding rescans, Siemens said. Both of the new BioMatrix sensors will also be available on Magnetom Vida. Magnetom Sola also utilizes the company's BioMatrix Tuners coil technology, which automatically adapts to challenging patient anatomy using its CoilShim and Slice Adjust techniques.
Magnetom Sola has a new magnet with higher homogeneity than the company's other 1.5-tesla scanner -- Magnetom Aera. It also offers a higher field-of-view -- 50 x 50 x 50 cm -- as well as a new gradient system and coils.
In addition to the BioMatrix technology, Magnetom Sola also comes with a number of other applications designed to speed up and automate routine exams. For example, its Simultaneous Multi-Slice technology, which can acquire multiple slices in parallel, can now be utilized in turbo spin-echo (TSE) sequences, Siemens said. As a result, the scanner can offer acquisition time savings of as much as 46% for musculoskeletal examinations, according to the company.
New clinical applications
The vendor has also incorporated its Compressed Sensing Cardiac Cine technique, which enables measurement of cardiac function in patients who can't hold their breath. The company's Compressed Sensing Grasp Vibe technique -- also migrated from Magnetom Vida -- is designed to provide push-button dynamic liver imaging under free-breathing, Siemens said.
Siemens has also incorporated the Whole-Body Dot Engine from Magnetom Vida. With this workflow engine, whole-body MRI scans can be performed in predictable time slots and in less than 30 minutes, the company said. Furthermore, an optional two-monitor user environment can enable users to begin postprocessing steps at the scanner prior to sending the studies over to the PACS.
The company said it has also integrated artificial intelligence (AI) techniques into Magnetom Sola for patient positioning, as well as various aspects of image acquisition and reconstruction. In addition, Siemens expects the ability of BioMatrix to derive quantitative information will enable it to serve as a platform for developing future applications powered by AI.
Magnetom Sola is currently being evaluated at the University Medical Center Mannheim. Siemens hopes to receive marketing clearance for Magnetom Sola in the summer in Europe, and to begin shipments in the third quarter. The system will complement the Magnetom Aera in the company's 1.5-tesla portfolio, according to the firm.
New Acuson Juniper platform
The company's ultrasound product launch is a system called Acuson Juniper that's designed to be a versatile product that is capable of thriving in a variety of demanding environments.
Acuson Juniper is the result of a two-year effort by Siemens to research the needs of its customer base, according to Miguel Trigueiros, global director of product marketing for ultrasound at the company's ultrasound headquarters in Mountain View, CA. The initiative involved over 350 participants and 170 workshops around the world, he said.
The fundamental issue driving the company's product development effort was a simple question to its users: If you designed an ultrasound scanner from the ground up, how would you design it to help you transform healthcare delivery?
Siemens therefore developed Juniper as an entirely new platform, designed to be versatile, adaptable, and transformative, and to meet the needs of users ranging from radiology to cardiology, even including disciplines such as interventional and ob/gyn imaging. The company picked Juniper as the product name due to the hardy plant's reputation for survival in some of the harshest environments, Trigueiros said.
"We wanted the name to tell the story of the product," Trigueiros told AuntMinnie.com. "Juniper is the world's most versatile tree; it will grow in mountains, deserts, your backyard. It adapts to cold and hot weather, and also it will grow in small spaces."
One example of Juniper's versatility is in how Siemens designed the system's transducers. Most ultrasound scanners have three or maybe four transducer ports, but Juniper sports six -- this makes it possible for users to have a wider array of probes available without having to unplug an old one and swap in a new one -- in fact, 80% of the clinical applications for which ultrasound is typically used can be covered with an active transducer, according to Trigueiros.
Siemens faced a conundrum with probe placement -- either on the left or right side of the system. In its workshops, the company didn't reach a consensus on which configuration was favored, so it simply built a control panel for Juniper that can be rotated 90°, enabling users to position the system with the ports to the side they prefer.
Other features on Juniper include a 21.5-inch LED display and a 13.3-inch touch display, as well as a gel warmer that's standard on the system rather than an option that customers have to pay for. There is also a USB port on the scanner so providers can transfer digital images to patients rather than having to output them to older media like prints or DVDs.
Siemens also took several steps to enhance Juniper's portability, an important factor for a shared-services scanner that might be moved around to different departments in a healthcare enterprise. The system has a battery and can be powered down in 3 to 5 seconds, and powered back up in five seconds.
Two other features include quiet operation -- Siemens made an effort to reduce Juniper's fan noise, so that it operates at a sound level of about 28 dB, some 48% quieter than the average for conventional scanners at 45 dB. For use in interventional environments, Siemens developed a rubber skin that can encase the unit during procedures and still enable sonographers to use the controls, then be removed and disinfected afterward -- an idea that Siemens received from the customer workshops, Trigueiros said.
Finally, Juniper features an entirely new architecture, including new signal processors and a new 128-channel beamformer that have led to big improvements in signal-to-noise ratio, faster image acquisition, and fewer artifacts from patient and probe motion. And all this in a compact package, with a footprint that is 36% smaller than the average ultrasound scanner. The scanner also supports teamplay, Siemens' peer-to-peer network, and EasyLink, a new remote service and diagnostics function.
Juniper has received both the CE Mark in Europe and 510(k) clearance from the U.S. Food and Drug Administration (FDA); the company will begin shipments in Europe and the U.S. after ECR 2018. Shipments in other global geographies are pending regulatory clearances, Trigueiros said.