VIENNA - Radiation dose from interventional radiology procedures continues to increase, and it's critical to have mechanisms to estimate and track dose accurately for both patients and equipment operators, according to a presentation on Sunday at ECR 2016.
In addition to accurate dose monitoring, dose thresholds for follow-up interventional radiology procedures should be considered in light of the high skin doses incurred during some procedures, said Andrew England, PhD, of the University of Salford in Manchester, U.K.
"Further minimization of radiation dose from [interventional radiology] procedures will require technical developments and some behavioral change," England said. He discussed the issues involving interventional radiology dose monitoring during a professional challenges session on Sunday morning.
Increase in dose
Although they offer numerous clinical benefits, interventional radiology (IR) procedures come with the disadvantage of radiation dose, both to patients and operators/staff, England said. The scope and frequency of interventional radiology procedures have grown rapidly over the past 10 years, and there are significant radiation dose implications.
For patients, the effective radiation dose can be high. In addition, the U.S. Food and Drug Administration (FDA) estimates that major radiation injury will happen between 1 in 10,000 to 1 in 100,000 interventional radiology procedures, England said.
Radiation dose is affected by the complexity of the procedure, the patient, the operator, and the equipment. This doesn't just concern the patients; the operator and staff also need to be monitored. For example, an operator with a workload of four procedures per day could have an annual dose of 10 mSv to 450 mSv to the neck, 10 mSv to 550 mSv to the eye lens, and 30 mSv to 640 mSv to the hand.
"[These doses] could exceed annual dose limits, and we know from the literature that they do," England said. "So the dose to patients and operators is significant."
Not surprisingly, dose management in interventional radiology has drawn a lot of attention lately, including a 2009 paper from the Society of Interventional Radiology (SIR) that provided guidelines for patient radiation dose management. In 2010, the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) and SIR also published a joint guideline on occupational radiation projection for interventional radiology. What's more, the U.S. National Council on Radiation Protection and Measurements (NCRP) has published guidance on radiation dose management for fluoroscopically guided interventional medical procedures.
Dose monitoring techniques
Traditionally, dose monitoring in interventional radiology involved calculating fluoroscopy time in minutes. This dose measurement surrogate had many limitations, however. An alarm that sounded at five minutes rarely affected behavior, and the fluoroscopy time calculation offers poor correlation with dose metrics, for example.
Another measure, kerma-area product (KAP), also known as dose-area product (DAP), calculated the product of the irradiated area and air kerma in terms of Gy/cm2. Also a surrogate of the amount of energy imparted to the patient, KAP offered reasonable correlations with stochastic risk and was useful for comparing procedures within and between institutions, England said.
"It's a very poor indicator of skin dose, however," he said. "And when you look at KAP values, it's very difficult to differentiate between a large field and a low-dose acquisition from a high dose and small-field acquisition."
KAP values can also be combined with dose-conversion coefficients -- derived from Monte Carlo calculations using phantoms -- to estimate effective dose.
More recently, the desire to provide cumulative patient dose data has resulted in the use of cumulative air kerma (CAK), also known as cumulative dose or reference-point dose, England said. This method calculates (in mGy) the total amount of energy deposited at a fixed point, usually 15 cm from the isocenter. CAK capability has been required on interventional radiology equipment since 2006, but it does suffer from a number of limitations, England said.
"It generally gives a poor indicator of skin dose and is only a single-point calculation," he said. "Depending on [a patient's] size and their position, the reference point and the skin point are not necessarily the same. This index is also measured in free air, so it doesn't take into account backscatter and attenuation, such as from the tabletop or the mattress."
In recent years, the calculation or estimation of skin dose has grown in importance, as this has a good relationship with deterministic risks, England said. One of the most useful metrics is peak skin dose (PSD), which measures the maximum radiation dose (in mGy) at any point on the patient's skin surface. PSD can predict the occurrence and severity of skin injuries, England said.
Measuring PSD directly can be difficult, as it would involve the use of thermoluminescent dosimeters (TLDs) or radiochromatic film. However, these don't provide real-time dose information, are expensive, and require medical physics expertise, England said.
Today, modern systems allow for real-time estimates of PSD and field-of-view PSD. These techniques, which take into account parameters such as kVP, tube current, filtration, field-of-view, pulse width, frame rate, and geometry, have an accuracy range of ± 20%.
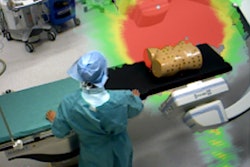
"Because it's an estimation and is based on simulations, you have to select some attributes of your patient before you start the examination," he said. "Then at the end of the procedure you can receive a dose report. Probably the most important thing is that you get a dose map showing the peak skin dose as a heat map on your patients."
Operator monitoring
Operators have traditionally used TLDs or film badges to monitor radiation dose exposure. While they can track accumulated dose, TLDs collect data over extended time periods and their data are often reported long after exposure.
"So it's very difficult to relate back to individual events or individual cases," he said. "They are good for monitoring and they are good for internal departmental reports, but using TLD or film data for operator dose monitoring rarely influences practice."
Another option is technology such as the DoseAware system (Philips Healthcare), which enables real-time operator dose monitoring and feedback from a wireless sensor worn above the lead apron at the level of the chest.
"It gives both the dose rate and cumulative dose with one-second resolution," he said.
Data can be captured from individual staff members, and the information includes date and time stamps to create a personalized dose history. Institutions have the ability to archive, report, and analyze the data, England said.
"What we've seen in the U.K. is that it does promote some initial culture change; people are aware of their operator doses," he said. "But my question would be: Are these culture changes temporary? We get a new toy, and often you think it has great value, but then as time goes on you may pay less attention to the dose-monitoring system."
There are also ethical and educational issues related to the use of these systems, according to England.
"One of the things we'd like to consider is the effects of these monitoring systems on procedural outcomes," he said.
Commercial standalone dose-management systems are also currently available, including DoseWatch (GE Healthcare) and Radimetrics (Bayer HealthCare). These can be used to document dose, benchmark performance, manage protocols, and identify outliers and patients at risk of significant injury.
"They are used postprocedurally, so whether they have any effect on the procedure at the time would be a question," England said. "But they can be used to generate alerts, so if a patient is referred for another IR procedure and they've had a significant skin dose, an alert can be raised before that patient can be scheduled."
Future of dose monitoring
England said that a comprehensive system is needed to assess patient dose intraprocedurally and in real-time.
"We need real-time feedback of operator/staff dose, for example, using the DoseAware system," he said. "We need to be able to integrate patient dose data from IR procedures into dose management systems such as Radimetrics or DoseWatch."
There's also the potential need for facilities to integrate dose information into local, national, and perhaps even international registries.
"But if we are to work hard with this dose data, it needs to be acted upon," he said.