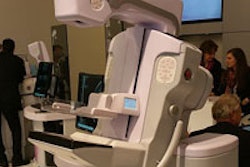
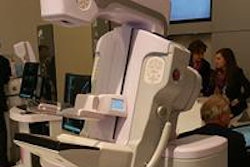
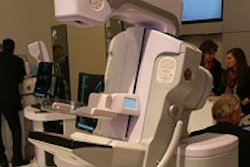
VIENNA - Mammography vendor Internazionale Medica Scientifica (IMS) is moving forward with plans to introduce a breast tomosynthesis system on the European market, the company announced at this week's European Congress of Radiology (ECR).
Called Giotto Tomo, the system is based on a C-arm design, a departure from the company's gantry ring-based Giotto full-field digital mammography (FFDM) system. The tomosynthesis unit will collect 13 exposures in a 40º arc around the patient's breast, with images then reconstructed into 3D volumes.
The company's technology differs from tomo units being developed by other vendors in that it features variable spacing between tomo slices, with more space at the edges of the arc and less in the center. This helps optimize imaging parameters and reduce radiation dose, according to Achille Albanese, marketing manager at IMS.
IMS is working with clinical sites in Italy and throughout Europe on a clinical trial for Giotto Tomo, which is already installed at a site in Bologna. In the trial, five sites in Europe will enroll a total of 800-850 patients, with the focus on comparing tomosynthesis to breast MRI for detecting additional cancers in women who already have malignant lesions.
Existing literature indicates that additional cancers can be found at a rate of 4% for the contralateral breast and 11% for the ipsilateral breast, making this a good potential application for tomo, according to Albanese. A second clinical trial will examine tomosynthesis for breast screening.
IMS expects to have a CE Mark for Giotto Tomo by April, and by 2012 plans to offer tomosynthesis on ring-based Giotto mammography systems as well. Albanese said IMS is preparing a 510(k) submission to the U.S. Food and Drug Administration for marketing clearance of Giotto as a conventional 2D FFDM system.