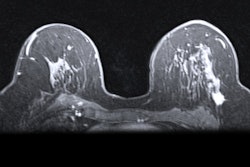
Disposable products developer Merit Medical Systems has received the CE Mark its Cianna Scout wire-free surgical guidance system.
Scout, which Merit acquired in 2018 with its purchase of Cianna Medical, helps radiologists and surgeons localize breast tumors. The system has already been cleared by the U.S. Food and Drug Administration (FDA) for breast tumor and soft tissue localization. Merit plans to roll out Scout in the European market in the coming weeks.
In other regulatory news, the company also reported that the FDA has granted BlueGrass Vascular Technologies a de novo classification order for BlueGrass's Surfacer Inside-Out Access catheter system. Merit is the distributor of the catheter system and owns 19% of BlueGrass.
In addition, Merit said it has received regulatory clearance from China to market the Swift Ninja microcatheter and InQwire Amplatz guidewire. The microcatheter and guidewire were approved by the country's National Medical Products Association.