Biomedical device company Biotronik has received CE approval for its new Edora pacemakers and cardiac resynchronization therapy pacemakers.
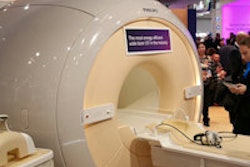
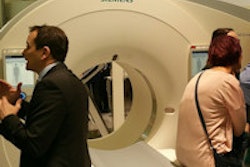
The devices feature the company's ProMRI technology, including the MRI AutoDetect sensor, which is designed to ensure the device is in its MRI-safe mode during only the period of the examination itself.
Edora single- and dual-chamber pacemakers currently have CE approval for full-body 1.5-tesla and 3-teals MRI examinations.