German nuclear medicine contrast company OctreoPharm Sciences has obtained orphan drug status for its SOMscan contrast agent for diagnosis of gastro-entero-pancreatic neuroendocrine tumors from the European Medicines Agency.
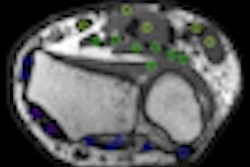
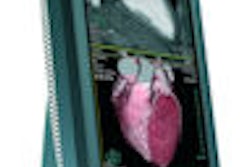
SOMscan is a gallium-68-labeled radioactive contrast agent used for PET examinations with the potential to detect neuroendocrine tumors in stage 1. It binds to four out of five specific tumor receptor subtypes on the surface of neuroendocrine tumors.
The orphan drug status entitles OctreoPharm Sciences to 10-year market exclusivity in Europe, following marketing approval for SOMscan. The designation also provides for special benefits, including possible exemptions in certain regulatory fees during development, the Berlin-based company said.