A photon-counting CT scanner called Naeotom Alpha and an MRI system aimed at underserved geographies were the highlight of a Siemens Healthineers media event on 16 November promoting products the company will be discussing at the RSNA 2021 meeting.
Naeotom Alpha first made waves in late September, when the U.S. Food and Drug Administration (FDA) announced it had granted clearance to the scanner, which it called the "first major imaging device advancement" in CT in nearly a decade. Photon-counting CT measures and processes data in a fundamentally different manner than conventional CT instrumentation, and it holds the promise of images with higher spatial resolution images at lower radiation dose. The scanner has also received the CE Mark.
 Siemens' new Naeotom Alpha photon-counting CT scanner. All images courtesy of Siemens Healthineers.
Siemens' new Naeotom Alpha photon-counting CT scanner. All images courtesy of Siemens Healthineers.At what it called its Shape 22 media event, Siemens Healthineers executives touted the benefits of its photon-counting CT technology, which is finally reaching the market after 15 years of development work. There are currently over 20 scanners in operation in the U.S. and Europe, most at university hospitals, according to company executives.
In contrast to the two-step process used in conventional CT systems to convert photon-counting x-rays into a medical image, Alpha's photon-counting technology utilizes a one-step conversion process, according to Philipp Fischer, head of CT. In this approach, the x-ray photons are converted into an electrical current that then generates the medical image.
"With this new technology, we are able to assess each and any single photon separately," said Philipp Fischer, head of CT. "And we are also able to assess the energy level of each and any single photon. And with that and with the removed one step of conversion we also have a higher dose efficiency intrinsically in that system."
To achieve this new conversion process, Siemens utilized cadmium telluride crystals in the photon-counting detector. The vendor said it was also able to eliminate electronic noise in the detector and image chain. Early work with the system indicates that eliminating this noise enables radiation dose to be cut by as much as 50%.
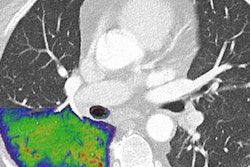
Siemens believes that photon-counting CT will have benefits across a wide range of clinical applications, but it sees cardiology as among the most exciting. Company executives note that between 15% to 30% of patients scheduled for coronary CT angiography (CCTA) aren't able to complete them due to coronary calcifications -- this requires them to be sent directly to invasive cardiac catheterization.
But Alpha is capable of acquiring virtual noncalcium images that show the "pure" lumen of the blood vessel, with no artifacts from calcium. This can enable heart centers to scan patients with calcium scores higher than what guidelines recommend, which can help reduce costs.
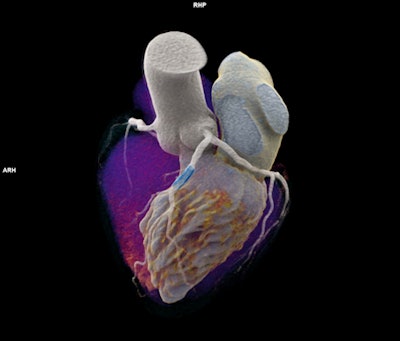 Naeotom Alpha's dual-source temporal resolution supports scanning of patients at any heart rate, while the photon-counting detector enables spectral information and high resolution, according to the vendor.
Naeotom Alpha's dual-source temporal resolution supports scanning of patients at any heart rate, while the photon-counting detector enables spectral information and high resolution, according to the vendor.Siemens is also highlighting the system's utility in oncology and pulmonology.
Alpha is a dual-source CT scanner with a rotation speed of 250 milliseconds and a temporal resolution of 66 milliseconds. The scanner is also capable of performing spectral CT.
In addition, Alpha is the vendor's first "digital-native" CT scanner, enabling remote operation, monitoring, and servicing. The system also includes Siemens' MyExam Companion artificial intelligence (AI)-based workflow software and is embedded in the firm's Syngo Carbon enterprise imaging platform, enabling access to postprocessing, advanced visualization capabilities, and integration with other clinically relevant data across modalities and input sources, Fischer said.
Furthermore, Siemens' AI Rad Companion software portfolio can be employed to automatically collect incidental findings on CT exams.
Siemens plans to show a mock-up of Alpha in its booth at RSNA 2021 and plans to begin a major commercial rollout of the system in the spring of 2022.
At the University Hospital Augsburg in German, Alpha has been in clinical use since April, scanning 150 to 200 patients per week and over 4,000 patients to date, according to Dr. Thomas Kröncke, director of the hospital's Clinic for Diagnostic and Interventional Radiology.
"Our experience covers a broad spectrum, from oncological cases to neuro, [musculoskeletal], cardiac, high-resolution CT of the chest, and geographic studies," he said. "The important thing to me is seeing better and seeing more due to the intrinsic spectral separation that photon counting allows."
The second major product launch for Siemens at RSNA 2021 will be Magnetom Free.Star, an MRI scanner designed to address underserved geographies and enable decentralized care, according to the vendor.
Free.Star is the second system in Siemens' Magnetom Free family, joining Free.Max, which was introduced in 2020. Offering a total cost of ownership that's up to 30% lower than previous systems, Free.Star features low siting requirements and a simplified workflow, said André Hartung, president of diagnostic imaging at Siemens.
"It's pretty much a push-button scanner," Hartung said. "So it's creating access for MRI technology to areas, geographies where MRI didn't play a role before because of affordability reasons, but at the same time pushes it into new clinical arenas."