A new European CT lung cancer screening trial is set to shed light on remaining questions surrounding the implementation of screening on a widespread basis, such as whether it is safe to extend screening intervals to two years. Data on the research were presented at ECR 2020.
The new trial is being led by Dr. Harry de Koning, professor and chair of public health at Erasmus Medical Center, who piloted the landmark Dutch-Belgian Randomized Lung Cancer Screening Trial (NELSON). Results from that trial were so positive that it has prompted follow-up work to guide more widespread implementation of CT lung cancer screening for high-risk populations.
De Koning's new trial is called the 4 in the Lung Run (4-iTLR) program, and its goal is to assess the relative safety -- or comparable detection of lung cancer stages I to III -- of a personalized risk-based and potentially less intensive screening regimen on the basis of a combination of two to three factors among individuals ages 60 to 79 at high risk for developing lung cancer: health risk factors, baseline CT scan result, and possibly biomarker outcomes.
"We hope to include throughout Europe 24,000 individuals with a baseline negative CT test result who have given consent, to be randomized into personalized screening (initially biennial screening) or annual standard screening," noted de Koning, speaking at the session "Lung cancer screening implementation in Europe: is it inevitable?" in a talk at ECR 2020.
The 4-iTLR trial follows on from the successful NELSON trial, the latest results of which de Koning also presented online, and which initially were published in New England Journal of Medicine on 29 January 2020.
Compellingly, the study revealed a 25% reduction in mortality. From 15,792 smoker or former smoker participants (85% of whom were male) 7,900 participants were randomized to the screening arm and underwent a series of CT scans at increasing intervals, while 7,892 in the control arm did not receive CT scans. Ten years after the first CT scan, the results of both groups were compared.
In the male cohort of the control group, 304 cancers were found. Of these, 13% were stage I and 46% were stage IV. In the screening group, 341 cancers were found, and of these, 60% were at stage I and only 10% were stage IV. Notably, 203 of these 341 cancers were detected in the first 5.5 years by CT, de Koning noted.
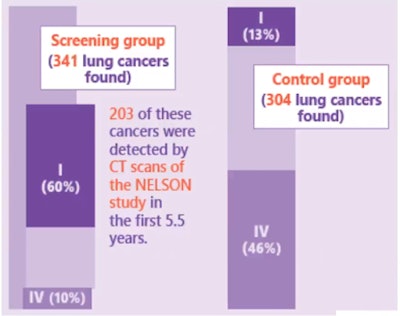 Stage shift in cancers found at 10 years. Comparison of screening arm versus control arm in NELSON male cohort. All images courtesy of Dr. Harry de Koning, Erasmus Medical Center.
Stage shift in cancers found at 10 years. Comparison of screening arm versus control arm in NELSON male cohort. All images courtesy of Dr. Harry de Koning, Erasmus Medical Center.The NELSON findings saw that CT lung screening had an important impact on lung cancer mortality between the control and screening groups at 10 years follow-up, with 2.5 deaths per 1,000 person-years in the screening group, 3.3 deaths per 1,000 person years in the control group, and a cumulative reduction rate (RR) of 0.76 (95% confidence interval [CI], 0.61-0.94), or around 25% reduced lung cancer mortality in males in the screening arm.
"In year 11 of the trial there was still a statistically significant reduction in lung cancer mortality rate of 0.78 (CI, 0.63-0.95) in the male cohort screening arm," de Koning noted. "For lung cancer screening, the evidence on effectiveness, benefits that outweigh the harms, and cost-effectiveness is now firm, and many suggested cost-effective lung cancer screening scenarios will give more benefits than any present cancer screening program," he said.
Personalized screening
Research is now needed in each country to assess the feasibility of fulfilling national requirements in practice. Part of this involved defining the maximum age range from a population or other risk registry and defining a risk cut-off for screening based on CT capacity.
De Koning pointed to the need for personalized risk calculators for individualized risk stratification. These individual, low cut-offs may yield better results than screening based on guidelines from the U.S. Preventive Services Task Force (USPSTF), but estimates indicate that the cost-effectiveness may touch $100,000 per quality-adjusted life year (QALY).
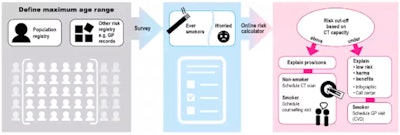 European screening policy should be guided by personalized risk stratification, according to de Koning.
European screening policy should be guided by personalized risk stratification, according to de Koning.Higher cut-offs and/or less intense but safe screening for risk groups seem needed for European policy-making, he said, adding that current pilot studies for CT lung cancer screening do not consider such regimens.
The new 4-iTLR trial should help to define the safety of two-year screening intervals and contribute to an overall implementation strategy to be developed through evaluating the comparative effectiveness and costs of personalized approaches in recruitment, volume CT lung cancer screening and follow-up, and smoking cessation and other comorbidity preventing strategies, de Koning noted.
Focusing on the future of screening, Dr. Fergus Gleeson from Oxford University Hospitals National Health Service (NHS) Foundation Trust presented a state-of-the-art overview of artificial intelligence (AI) trends. Gleeson asked ECR 2020 viewers what they would wish for in their perfect computer-aided detection (CAD) algorithm, listing what he would have in his.
Ideally, the perfect lung screening CAD would take into account all the different patient types, the pathways through which they had been scanned and the nodules detected, whether contrast was used, how the images were reconstructed, the resolution involved, and whether there were prior scans that showed or didn't show the nodule and what size it was.
"You'd want it to have a high sensitivity and specificity, to be able to diagnose benign or malignant nodules, potentially the cell type, take into account second malignancies and other significant benign disease such as rheumatoid arthritis," he noted. "It would ... take into account the clinical data, the type of technology used, and also be able to manage future technological advances."
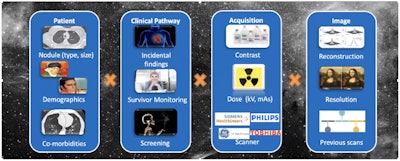 Computer-aided detection in the future will take into account many other factors besides nodule size and location, according to Dr. Fergus Gleeson. Image courtesy of Dr. F. Gleeson, Oxford University Hospitals National Health Service (NHS) Foundation Trust.
Computer-aided detection in the future will take into account many other factors besides nodule size and location, according to Dr. Fergus Gleeson. Image courtesy of Dr. F. Gleeson, Oxford University Hospitals National Health Service (NHS) Foundation Trust.CAD accuracy has improved significantly in recent years and current thinking is that such programs should be used in CT lung cancer screening.
"It isn't perfect, though, and humans do detect the nodules that are missed by CAD. So although it's got better, we're not redundant yet in terms of nodule detection," he said.
He flagged how CAD is not used in isolation and pointed to a future in which doctors will not just take into account clinical data and history but also blood biomarkers -- whether circulating tumor DNA, proteomics, or metabolomics.
A number of current multicenter studies are examining how AI and CAD combined with proteomics and metabolomics can enable doctors to get closer to diagnosis of benign disease or malignancy and potentially even cell type. With this in mind, structured machine learning using radiomics has been used relatively recently to be able to determine cell type and the degree of invasion, particularly useful in semisolid and ground-glass nodules, according to Gleeson.
Prospective analysis
While retrospective data on AI is growing, prospective data remains scarce. This may change with reportedly the world's first prospective multicenter trial of an imaging-based lung cancer prediction AI. The algorithm has been developed by Optellum, an Oxford lung cancer AI solutions company, which is currently undertaking the Artificial Intelligence and Big Data for Early Lung cancer diagnosis (IDEAL) study.
The IDEAL trial involves 1,500 patients, the first of whom were enrolled in August 2018, with at least 1,050 consecutive patients following with standard of care. The trial aims to determine if the algorithm will reduce the number of false positives and negatives, and the number of biopsies and PET scans needed to establish a diagnosis.
Such algorithms will be used in screening not just for nodule detection but also to look at the lungs and heart to check for nonmalignant lung and cardiac disease, to identify regional variation in ventilation, and for stratifying patients' coronary artery – as well as cardiovascular disease in unenhanced scans, Gleeson continued.
"While AI has a future, we're required to be part of it. The idea that we are going to be replaced by AI is at present incorrect, and actually what is going to be replaced is radiologists who don't use it," he noted.