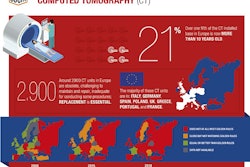
The European Coordination Committee of the Radiological, Electromedical, and Healthcare IT Industry (COCIR) is calling on the European Commission to take action to eliminate the backlog of harmonized standards for medical devices and to reinstall a sustainable and efficient process for harmonizing standards.
In its position paper, COCIR offered six recommendations to get the scheme of harmonized standards to be functioning and up to date again in the European Union:
- Speed up approval of the standardization request.
- Ensure a flexible standardization request.
- Realize a flexible publication schedule for the Official Journal of the European Union.
- Streamline the assessment of Annexes Z.
- European regulators -- including the European Commission -- should actively participate in standards development.
- Provide for a process for swift harmonization of standards under the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR).
"The processes for drafting and adopting a Standardization Request as well as harmonizing and referencing standards in the Official Journal have clearly become too burdensome," COCIR wrote in the position paper. "Not only standardizers but also the European Commission requires lighter procedures with fewer administrative checks. Such lighter procedures can speed up the harmonization process and still provide clarity on the (legal) coverage of the essential requirements."