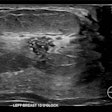
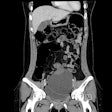
The U.S. Food and Drug Administration (FDA) has issued a recall of Hologic’s BioZorb 3D bioabsorbable radiographic markers, with 252 injuries reported to date.
The recall follows a warning by the FDA to consumers, healthcare providers, and healthcare facilities not to use the markers as well as a voluntary recall by Hologic, both issued in October. This recall involves removing the devices from where they are used or sold, as the devices may cause serious injury or death with continued use, the FDA said.
“Monitor patients who have an implanted BioZorb Marker for signs of any adverse events,” the FDA advised, in an 18 December notification.
In addition, facilities should quarantine all products and share the notification with all surgeons who have used or plan to use the BioZorb marker, the FDA said.
Hologic said that in February, it began receiving patient complaints describing complications and adverse events associated with the devices that included serious injuries such as pain, infection, rash, device migration, device erosion, seroma, discomfort, and other complications from feeling the device in the breast, and in some limited instances, additional medical treatment.
Customers in the U.S. with questions about this recall should contact Hologic at [email protected], the FDA said.