Shear-wave elastography (SWE) could be used as an indicator of the risk of breast implant rupture, a study published on 20 January in the Journal of Biomechanics.
Researchers led by Dr. Laetitia Ruffenach from the University of Strasbourg in France reported that elastography can visualize breast implant stiffness as implants degrade over time, measuring rupture risk and helping diagnose replacements for implants.
“By observing the consequences of the physical-chemical mechanisms at work within patients, this study shows that ultrasound elastography could be used in vivo as a quantitative indicator of the risk of breast implant rupture and help diagnose their replacement,” Ruffenach and colleagues wrote.
Breast implants are used after breast cancer resection, and they must be changed regularly to avoid rupturing. However, the researchers noted that there are no quantitative criteria to help guide when and how they should be replaced. They also suggested that the mechanical evolution of the gels and membranes within the implants could play a role in early rupturing.
While elastography has been used in supplemental imaging to diagnose breast cancer, its use in assessing breast implants has also been explored. This includes evaluating the impact of implants on surrounding body tissues, such as inflammation, capsular contracture, fibrosis, and hematoma.
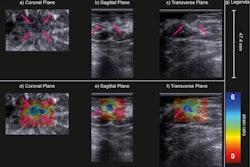
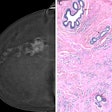
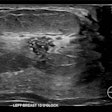
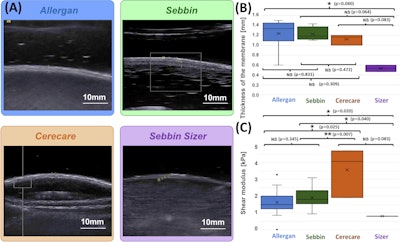 (A) Examples of B-mode images for the measurement of membrane thickness of implants from each of the four types of implants. Images are presented at the same scale. Membrane thicknesses (B) and then shear moduli measured by ARFI method (C) are compared without dissociation as a function of prosthesis age or implantation time. (NS: p-value > 0.05, *: p-value < 0.05, **: p-value < 0.01, ***: p-value < 0.001). Images are available for republishing under a Creative Commons license (CC BY-NC-ND 4.0 Deed) and courtesy of the Journal of Biomechanics.
(A) Examples of B-mode images for the measurement of membrane thickness of implants from each of the four types of implants. Images are presented at the same scale. Membrane thicknesses (B) and then shear moduli measured by ARFI method (C) are compared without dissociation as a function of prosthesis age or implantation time. (NS: p-value > 0.05, *: p-value < 0.05, **: p-value < 0.01, ***: p-value < 0.001). Images are available for republishing under a Creative Commons license (CC BY-NC-ND 4.0 Deed) and courtesy of the Journal of Biomechanics.
Ruffenach and co-authors used SWE on nonruptured breast implants and studied the evolution of mechanical properties exhibited by the implants over time.
In total, the team included data from 35 breast explants that had been implanted in women for up to 17 years. It evaluated four distinct brands of breast implants: Allergan, Sebbin, Sebbin Sizer, and Cerecare. It also quantified gel stiffness using virtual touch quantification, an SWE method based on acoustic radiation force impulse imaging.
Elastography showed that while the Allergan and Sebbin brands have similar stiffness (1.79 kPa and 1.9 kPa, respectively), the Cerecare implants appeared stiffer at 3.57 kPa. The Sebbin Sizer implants had the lowest stiffness at 0.8 kPa.
“We can assume that the manufacturing processes of the Sebbin and Sebbin Sizer have an influence on the gel stiffness, as the latter are not intended for long-term resistance,” the researchers wrote.
Also, all explants in the study showed a significant increase in stiffness in time after about eight years of implantation. The team highlighted that an explant carried for 210 months is three times stiffer than for 38 months.
Finally, the researchers used matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI) analysis to find out whether organic compounds from biological breast tissues surrounding implants were likely to be found in the gel of the explants.
They found phosphoglycerides on the membranes and metabolites of cholesterol from the breast tissues, arranged homogeneously within the gel. This means that organic molecules were able to cross the membranes and diffuse within the gel.
The study authors highlighted that their study “opens the way for an understanding of the mechanical phenomena leading to the rupture of the implants. They added that it could also open the possibility of using noninvasive elastography to predict rupture risk and diagnose the need for implant replacement.
The study can be found in its entirety here.